Earnings summaries and quarterly performance for QUEST DIAGNOSTICS.
Executive leadership at QUEST DIAGNOSTICS.
James E. Davis
Chief Executive Officer and President
Catherine T. Doherty
Executive Vice President, Regional Businesses
Karthik Kuppusamy
Senior Vice President, Clinical Solutions
Michael E. Prevoznik
Senior Vice President, General Counsel
Sam A. Samad
Executive Vice President, Chief Financial Officer
Board of directors at QUEST DIAGNOSTICS.
Denise M. Morrison
Director
Gary M. Pfeiffer
Director
Luis A. Diaz, Jr., M.D.
Director
Robert B. Carter
Director
Timothy L. Main
Director
Timothy M. Ring
Lead Independent Director
Tracey C. Doi
Director
Vicky B. Gregg
Director
Wright L. Lassiter III
Director
Research analysts who have asked questions during QUEST DIAGNOSTICS earnings calls.
Elizabeth Anderson
Evercore ISI
8 questions for DGX
Erin Wright
Morgan Stanley
8 questions for DGX
Jack Meehan
Nephron Research LLC
8 questions for DGX
Kevin Caliendo
UBS
8 questions for DGX
Patrick Donnelly
Citi
8 questions for DGX
Michael Cherny
Leerink Partners
7 questions for DGX
Andrew Brackmann
William Blair & Company, L.L.C.
6 questions for DGX
David Westenberg
Piper Sandler
6 questions for DGX
Luke Sergott
Barclays
6 questions for DGX
Michael Ryskin
Bank of America Merrill Lynch
6 questions for DGX
Pito Chickering
Deutsche Bank
6 questions for DGX
Tycho Peterson
Jefferies
5 questions for DGX
Andrew Brackman
William Blair
2 questions for DGX
Ann Hynes
Mizuho Financial Group
2 questions for DGX
Benjamin Shaver
Deutsche Bank AG
2 questions for DGX
Eric Caldwell
Baird
2 questions for DGX
Noah Kava
Jefferies
2 questions for DGX
Aaron David Izen
Bank of America
1 question for DGX
Anna Krasinski
Barclays
1 question for DGX
Eugene Park
Robert W. Baird & Co. Incorporated
1 question for DGX
Lisa Gill
JPMorgan Chase & Co.
1 question for DGX
Meghan Holtz
Jefferies Financial Group Inc.
1 question for DGX
Michael Cherney
Jefferies Financial Group Inc.
1 question for DGX
Stephanie Davis
Barclays
1 question for DGX
Recent press releases and 8-K filings for DGX.
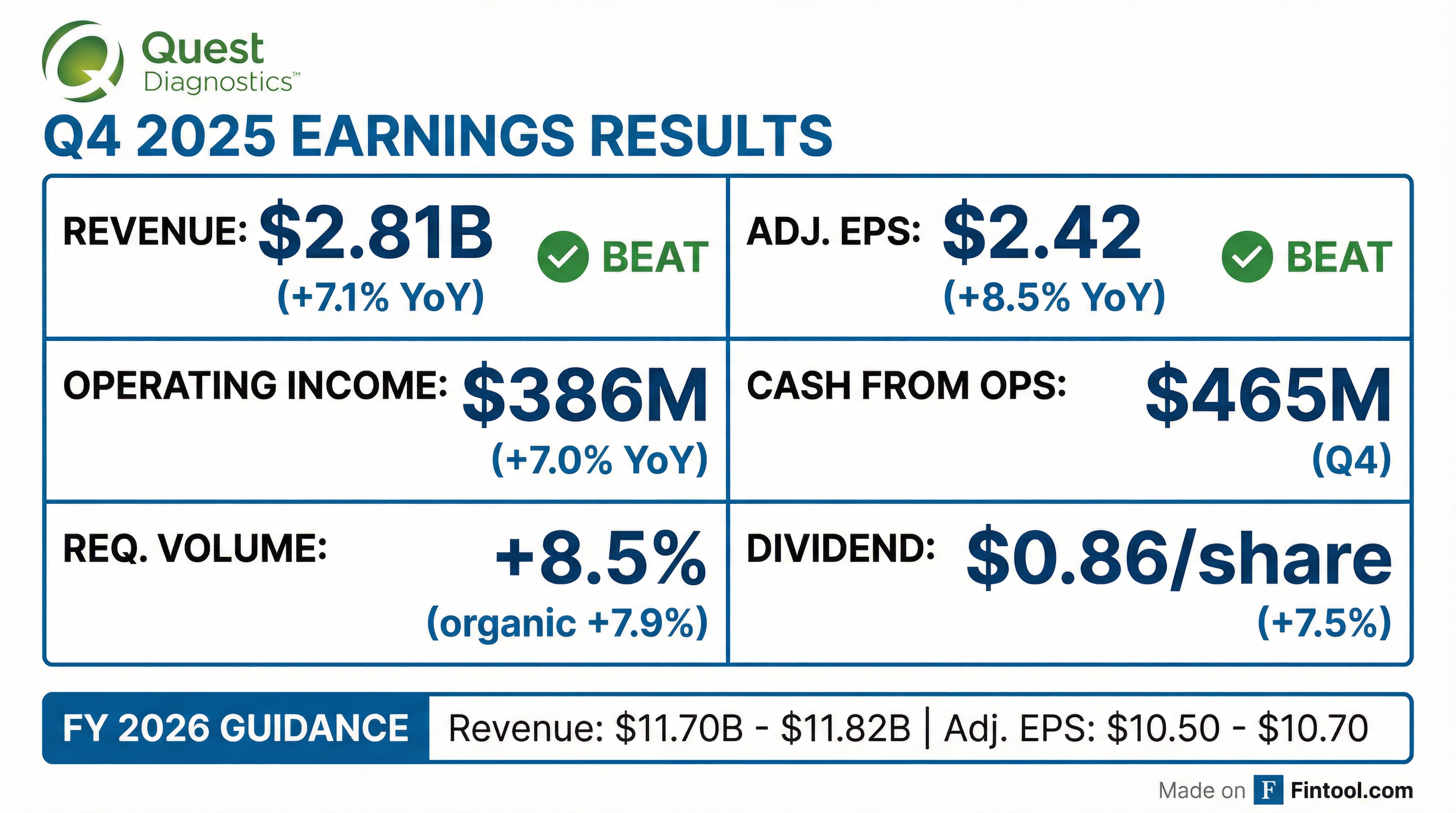
- Quest Diagnostics reported Q4 2025 net revenues of $2.806 billion, up 7.1% year-over-year.
- Q4 2025 adjusted operating margin was 15.3%, down from 15.6% in Q4 2024.
- Q4 2025 adjusted diluted EPS was $2.42, an 8.5% increase year-over-year.
- Full-year 2026 guidance calls for revenues of $11.70 billion–$11.82 billion and adjusted diluted EPS of $10.50–$10.70.
- Q4 consolidated revenues of $2.81 billion, up 7.1% year-over-year; organic revenue growth of 6.4% and requisition volumes up 8.5%.
- Full-year 2025 delivered double-digit growth in revenues and earnings per share.
- 2026 guidance: revenues of $11.7 billion–$11.82 billion (+6.0%–7.1%), adjusted EPS of $10.50–$10.70, and cash from operations of ~$1.75 billion.
- Co-lab solutions expected to generate $1 billion in annual revenues in 2026; partnership with Corewell Health will add $250 million of organic revenue at low single-digit margins.
- Consumer-initiated testing grew >20% in 2025, delivering ~$250 million in revenues; QuestHealth.com expanded to over 150 tests.
- Q4 2025 revenues were $2.81 billion (+7.1% YoY; organic +6.4%), total volume +8.5%, with adjusted EPS of $2.42 (+8.5% YoY).
- 2025 full year delivered double-digit revenue and EPS growth; cash from operations was $1.89 billion.
- 2026 guidance: revenues of $11.7 billion–$11.82 billion (+6.0%–7.1%), adjusted EPS $10.50–$10.70, cash from operations ~ $1.75 billion, and capex ~ $550 million; operating margin is expected to expand.
- Strategic initiatives include scaling co-lab solutions with Corewell Health (targeting $1 billion in annual revenues in 2026) and growing consumer-initiated testing (nearly $250 million in 2025 via QuestHealth.com and wellness partnerships).
- Q4 2025 revenues were $2.81 billion, up 7.1% YoY, with organic revenue growth of 6.4%, and adjusted EPS of $2.42 (vs. $2.23 prior year).
- Full-year 2025 delivered double-digit growth in both revenues and EPS, driven by clinical innovations, strategic collaborations, and operational enhancements.
- 2026 guidance calls for revenues of $11.70 – $11.82 billion (6.0%–7.1% growth), adjusted EPS of $10.50 – $10.70, operating margin expansion, cash from operations of ~$1.75 billion, and CapEx of ~$550 million.
- Strategic initiatives include a one-year delay of PAMA rate cuts through end 2026, 3% cost savings via the Invigorate program, and expected $250 million in low-single-digit-margin co-lab revenues from Corewell Health in 2026.
- Q4 2025 revenues of $2.81 billion (+7.1% y/y); reported diluted EPS of $2.18 (+11.8% y/y) and adjusted diluted EPS of $2.42 (+8.5% y/y)
- FY 2025 net revenues of $11.04 billion (+11.8% y/y); reported diluted EPS of $8.75 (+13.8% y/y); adjusted diluted EPS of $9.85 (+10.3% y/y); cash from operations of $1.89 billion
- 2026 guidance: revenues of $11.70–11.82 billion, reported diluted EPS of $9.45–9.65, and adjusted diluted EPS of $10.50–10.70
- Quest Diagnostics’ Board approved a 7.5% increase in its quarterly cash dividend to $0.86 per share (annualized $3.44), payable April 20, 2026, to shareholders of record April 6, 2026.
- The Board also increased its share repurchase authorization by $1 billion, adding to the roughly $0.4 billion available under the existing program as of December 31, 2025.
- Quest Diagnostics expanded network access with Elevance and secured partnerships with large corporate physician groups to drive growth in hospital labs, reference testing, collaborative lab solutions and life sciences services.
- Consumer-initiated testing grew from $30 million in 2021 to a $250 million run rate by Q4, with QuestHealth.com contributing $100 million and channel partners (e.g., Function Health, Whoop) adding $150 million.
- Focused on advanced diagnostics—including cardiometabolic, autoimmune and brain health—with commercial launch of the AB4240 Alzheimer's blood-based test for amyloid and tau biomarkers.
- Reaffirmed 2026 guidance: 4–5% total revenue growth, 75–150 bps operating margin expansion, 7–9% adjusted EPS growth, and $3–3.3 billion free cash flow after capex.
- Tests one-third of U.S. adults, completing ~220 million requisitions and directly drawing blood for ~80 million individuals in 2024; network spans 8,000 sites and covers 90% of lives with 80 billion data points ( ).
- Prevention and wellness represent ~33% of revenue; consumer-initiated testing surged from $30 million in 2021 to a $250 million run rate in 2025, led by QuestHealth.com’s $100 million business ( ).
- Operates in a $90 billion laboratory market (~2% of a $5 trillion healthcare spend); targets 4–5% organic revenue growth, 75–150 bps margin expansion, 7–9% EPS growth, and $3–3.3 billion free cash flow (2025–27) with capital allocated to M&A, dividends, and share repurchases ( ).
- $2.8 billion deployed in M&A over three years—including the $1 billion LifeLabs Canada acquisition—and growing co-lab hospital services from $800 million toward a $1 billion target by 2026 ( ).
- Quest Diagnostics tested one-third of the U.S. adult population in 2024 with 217 million requisitions and 80 million blood draws, covering 110 million unique patients across 8,000+ locations.
- Prevention and wellness represents ~1/3 of revenue, while the consumer business rose from $30 million in 2021 to a $250 million run rate in Q4 2025 via QuestHealth.com ($100 million) and channel partners ($150 million).
- The company reaffirmed its 2026 outlook: 4–5% revenue growth, 75–150 bps operating margin expansion, 7–9% adjusted EPS growth, and $3–3.3 billion free cash flow (2025–2027) targets.
- Key investments include AI/automation (end-to-end lab systems, digital cytology), $2.8 billion in M&A (2023–25), and Project Nova to modernize the order-to-cash infrastructure.
- Quest Diagnostics and Corewell Health complete laboratory services joint venture, Diagnostic Lab of Michigan, LLC, with Quest owning 51% and Corewell Health 49%
- Existing Quest and Corewell laboratories will continue providing services until a new 100,000-square-foot facility opens in Q1 2027
- Quest begins deploying Co-Lab Solutions across 21 Corewell Health hospitals, including supply chain, lab management, patient blood and anemia management, and lab analytics and stewardship
Quarterly earnings call transcripts for QUEST DIAGNOSTICS.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more