Earnings summaries and quarterly performance for MoonLake Immunotherapeutics.
Executive leadership at MoonLake Immunotherapeutics.
Board of directors at MoonLake Immunotherapeutics.
Research analysts covering MoonLake Immunotherapeutics.
Recent press releases and 8-K filings for MLTX.
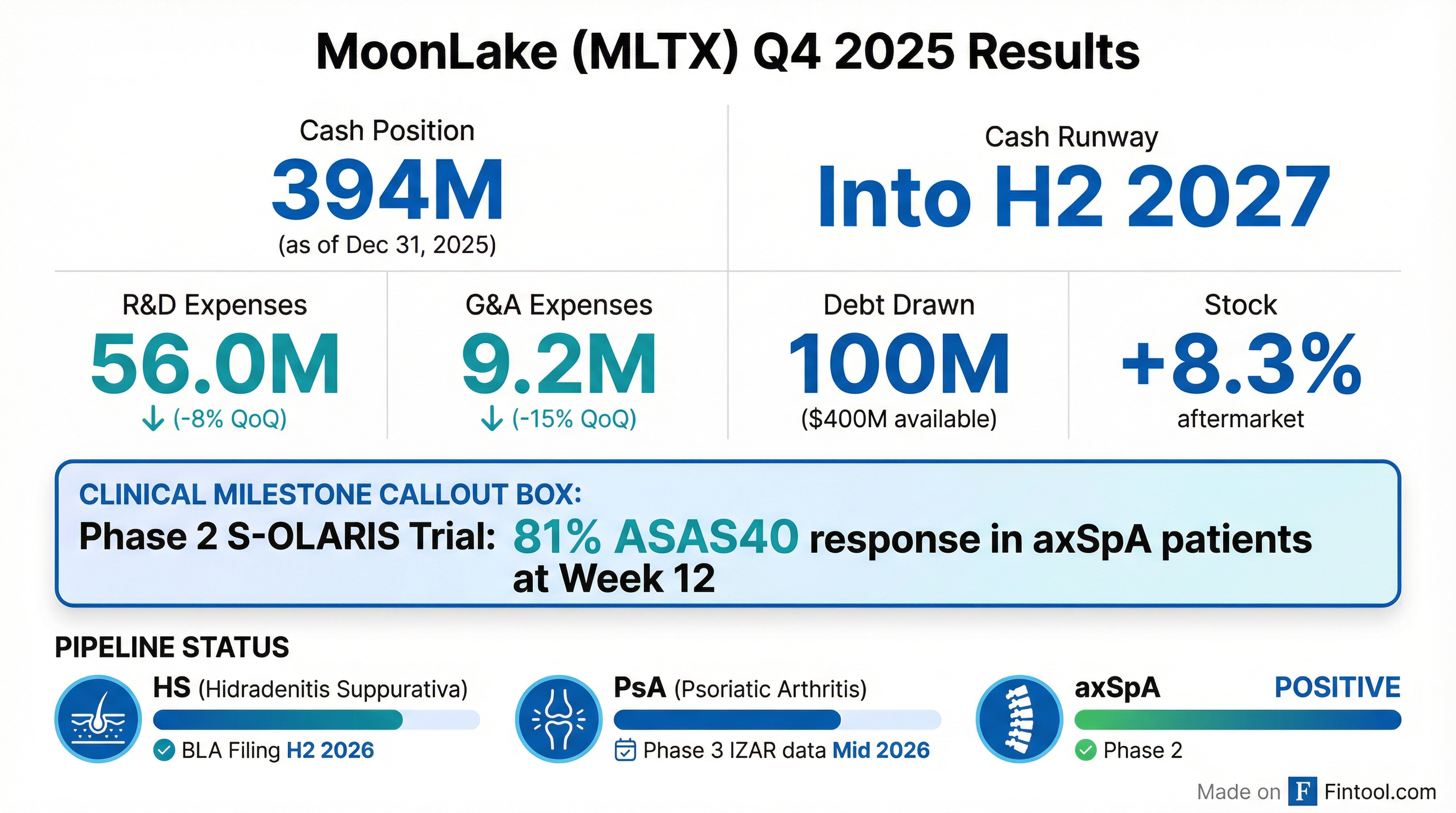
MoonLake Reports Positive Phase 2 Trial Results and 2025 Financials
MLTX
Earnings
New Projects/Investments
Guidance Update
- MoonLake Immunotherapeutics announced positive topline results from its Phase 2 S-OLARIS clinical trial for sonelokimab (SLK) in axial spondyloarthritis (axSpA), demonstrating clinically meaningful benefit with 81% of patients achieving ASAS40 by Week 12.
- As of December 31, 2025, MoonLake reported $394.0 million in cash, cash equivalents, and short-term marketable debt securities, which, together with funds from its latest equity raise, are expected to provide a cash runway into the second half of 2027.
- The company also announced an amendment to its debt facility with Hercules Capital, including a concurrent drawdown of $25 million and up to $400 million in non-dilutive funds remaining available to support future funding needs.
- For the fourth quarter ended December 31, 2025, research and development expenses were $56.0 million, and general and administrative expenses were $9.2 million.
Feb 22, 2026, 4:00 PM
MoonLake Immunotherapeutics receives positive FDA feedback on sonelokimab BLA pathway
MLTX
New Projects/Investments
Takeover Bid
Guidance Update
- The U.S. FDA indicated that existing data for sonelokimab (SLK) in hidradenitis suppurativa (HS) may provide "substantial evidence of effectiveness" to support a Biologics License Application (BLA) without requiring additional pivotal HS trials.
- MoonLake Immunotherapeutics (MLTX) plans to continue BLA preparations, targeting submission in the second half of 2026.
- Following the announcement, MLTX shares surged roughly 27–32% on heavy trading, as the regulatory clarity removed a major near-term uncertainty.
- Investors should consider MoonLake's lack of product revenue, sizable recent losses (approximately US$210.50 million in the last reported year), and associated funding and dilution risks.
- Reports also noted that Merck previously approached MoonLake with a bid exceeding $3 billion, highlighting industry interest in sonelokimab.
Jan 8, 2026, 2:31 PM
MoonLake Immunotherapeutics Announces Positive FDA Feedback for Sonelokimab in HS and Investor Day
MLTX
New Projects/Investments
Guidance Update
- MoonLake Immunotherapeutics received positive feedback from the U.S. FDA regarding its clinical evidence strategy for Sonelokimab (SLK) in Hidradenitis Suppurativa (HS).
- The FDA confirmed that MoonLake may establish substantial evidence of effectiveness (SEE) for SLK in HS without additional clinical trials, utilizing data from its existing VELA-1, VELA-2, and MIRA trials.
- Based on this feedback, MoonLake plans to submit a Biologic License Application (BLA) for SLK in HS in H2 2026.
- An Investor Day is scheduled for February 23, 2026, to further discuss the FDA feedback and present new clinical data for SLK across indications.
Jan 8, 2026, 1:00 PM
MoonLake Immunotherapeutics Faces Securities Class Action Lawsuit
MLTX
Legal Proceedings
- A securities class action lawsuit has been filed against MoonLake Immunotherapeutics (MLTX), with a Lead Plaintiff Deadline of December 15, 2025.
- The lawsuit alleges that MoonLake made materially false and misleading statements regarding the superiority of its Nanobody technology for its drug candidate, sonelokimab (SLK).
- These alleged misrepresentations led to a 90% stock crash on September 29, 2025, after the VELA-2 trial results showed the drug's efficacy was inferior to a competitor.
- The class period for investors who purchased MLTX stock and suffered losses is March 10, 2024, to September 29, 2025.
Dec 11, 2025, 5:53 PM
MoonLake Immunotherapeutics Faces Securities Class Action Lawsuit
MLTX
Legal Proceedings
Profit Warning
- Hagens Berman is leading a securities class action lawsuit against MoonLake Immunotherapeutics, Inc. (MLTX), with a lead plaintiff deadline of December 15, 2025.
- The lawsuit alleges that MoonLake misrepresented SLK's clinical performance and its competitive differentiation from BIMZELX, specifically questioning whether its Nanobody structure provided superior efficacy in Phase 3 VELA trials.
- This alleged misrepresentation led to a 90% stock plunge on September 29, 2025, with shares falling from $61.99 to $6.24.
- The Class Period for investors who purchased MLTX shares and suffered substantial losses is March 10, 2024, through September 29, 2025.
Dec 5, 2025, 1:06 PM
MoonLake Immunotherapeutics Faces Securities Class Action Lawsuit
MLTX
Legal Proceedings
Demand Weakening
- Hagens Berman has filed a securities class action lawsuit against MoonLake Immunotherapeutics (MLTX), alleging the company concealed material adverse facts regarding the clinical performance of its drug SLK and its ability to differentiate from competitors like BIMZELX.
- The lawsuit claims MoonLake misrepresented that SLK's distinct Nanobody structure would provide superior clinical efficacy, failing to disclose that it did not confer a meaningful clinical advantage in the Phase 3 VELA trials.
- As a result of these alleged undisclosed trial flaws, MLTX's stock experienced a 90% loss, falling from $61.99 to $6.24 on September 29, 2025.
- Investors who purchased MLTX shares during the Class Period, from March 10, 2024, through September 29, 2025, and suffered substantial losses, are encouraged to contact Hagens Berman, with the lead plaintiff deadline set for December 15, 2025.
Dec 3, 2025, 8:05 PM
MoonLake Immunotherapeutics Faces Securities Class Action Lawsuit
MLTX
Legal Proceedings
Demand Weakening
- Hagens Berman is investigating MoonLake Immunotherapeutics (MLTX) regarding alleged undisclosed facts about SLK's clinical performance and its ability to differentiate from competitors like BIMZELX.
- The lawsuit focuses on an alleged gap between MoonLake's optimistic public statements and the undisclosed reality of SLK's performance in the Phase 3 VELA trials.
- Allegations include the company's failure to disclose that SLK and BIMZELX share the same molecular targets (interleukin-17, or IL-17), and misrepresenting that SLK's Nanobody structure would provide superior clinical efficacy.
- MLTX stock experienced a 90% loss, falling from $61.99 to $6.24 on September 29, 2025.
- The deadline for investors to move the Court for appointment as lead plaintiff in the securities class action lawsuit is December 15, 2025.
Dec 2, 2025, 1:38 AM
MoonLake Immunotherapeutics' Stock Crashes and Faces Lawsuit
MLTX
Legal Proceedings
Profit Warning
- MoonLake Immunotherapeutics' stock crashed nearly 90% in a single day, falling from $61.99 to $6.24, following the release of disappointing Phase 3 trial results for its lead and only drug candidate, sonelokimab (SLK).
- The VELA-2 Phase 3 trial failed to meet its primary endpoint, and the efficacy results for SLK were noted to be substantially lower than those previously achieved by the competitor drug, BIMZELX.
- A securities fraud class action lawsuit has been filed, alleging MoonLake made materially false and misleading statements to investors about SLK's clinical prospects and exaggerated its benefits over competitors.
- The lawsuit's Class Period is from March 10, 2024, to September 29, 2025, with a Lead Plaintiff Deadline of December 15, 2025.
Nov 28, 2025, 10:50 PM
MoonLake Immunotherapeutics' Stock Crashes 90% Following Disappointing Phase 3 Trial Results, Triggers Lawsuit
MLTX
Legal Proceedings
Profit Warning
- MoonLake Immunotherapeutics' (MLTX) stock crashed nearly 90% on September 28, 2025, after its lead and only drug candidate, sonelokimab (SLK), reported disappointing Phase 3 trial results.
- The VELA-2 Phase 3 trial for SLK failed to meet its primary endpoint, and its efficacy results were substantially lower than those of a competitor drug, BIMZELX.
- A securities fraud class action lawsuit has been filed, alleging that MoonLake and certain executives made materially false and misleading statements about SLK's clinical prospects and exaggerated its benefits.
- The class period for the lawsuit is from March 10, 2024, to September 29, 2025, with a lead plaintiff deadline of December 15, 2025.
Nov 26, 2025, 1:50 PM
MoonLake Immunotherapeutics Faces Lawsuit After Disappointing Drug Trial Results
MLTX
Legal Proceedings
Profit Warning
- MoonLake Immunotherapeutics (MLTX) stock plummeted nearly 90% on September 28, 2025, following the release of disappointing Phase 3 trial results for its lead and only drug candidate, sonelokimab (SLK).
- The VELA Phase 3 trials for SLK, intended to treat moderate to severe hidradenitis suppurativa, included the VELA-2 trial which failed to meet its primary endpoint, and efficacy results were substantially lower than a competing drug, BIMZELX.
- A securities fraud class action lawsuit has been filed against MoonLake and certain executives, alleging they made materially false and misleading statements about SLK's clinical prospects and exaggerated its benefits.
- The stock price fell by $55.75 per share, from $61.99 to $6.24, in the trading day after the news.
Nov 25, 2025, 9:31 PM
Fintool News
In-depth analysis and coverage of MoonLake Immunotherapeutics.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more