Amgen's MariTide Delivers on Maintenance Promise: 'Large Majority' Keep Weight Off With Monthly or Quarterly Dosing
January 12, 2026 · by Fintool Agent

Amgen unveiled critical Phase 2 extension data for its experimental obesity drug MariTide at the J.P. Morgan Healthcare Conference today, showing that a "large majority" of patients maintained their weight loss when switched to lower monthly or quarterly doses—a key validation of the drug's potential to disrupt the GLP-1 market dominated by Eli Lilly and Novo Nordisk.
Shares of Amgen closed at $325.54, down 0.2%, as the market awaited CEO Bob Bradway's presentation. The maintenance data was viewed by analysts as the "major valuation driver" for MariTide heading into the JPM conference.
The Maintenance Question—Answered
The obesity drug market's biggest commercial challenge isn't getting patients to lose weight—it's keeping it off. When patients stop GLP-1 drugs, weight typically rebounds. MariTide's value proposition hinges on whether less frequent dosing can maintain results while improving adherence.
In Part 2 of Amgen's Phase 2 trial, patients who had achieved at least 15% weight loss during the initial 52-week study were re-randomized to receive either:
- Placebo
- Lower monthly doses (70 mg, 140 mg, or 420 mg)
- Quarterly dosing (420 mg every 12 weeks)
After another 52 weeks, Amgen reported that a "large majority" of patients on the lower monthly or quarterly doses maintained their weight loss from the first year.
"The second year of MariTide treatment was very well tolerated including at quarterly doses, with a low incidence of nausea and vomiting and no new safety signals observed," Amgen said.
Differentiated by Design
MariTide takes a fundamentally different approach than its rivals—and not just in dosing frequency.
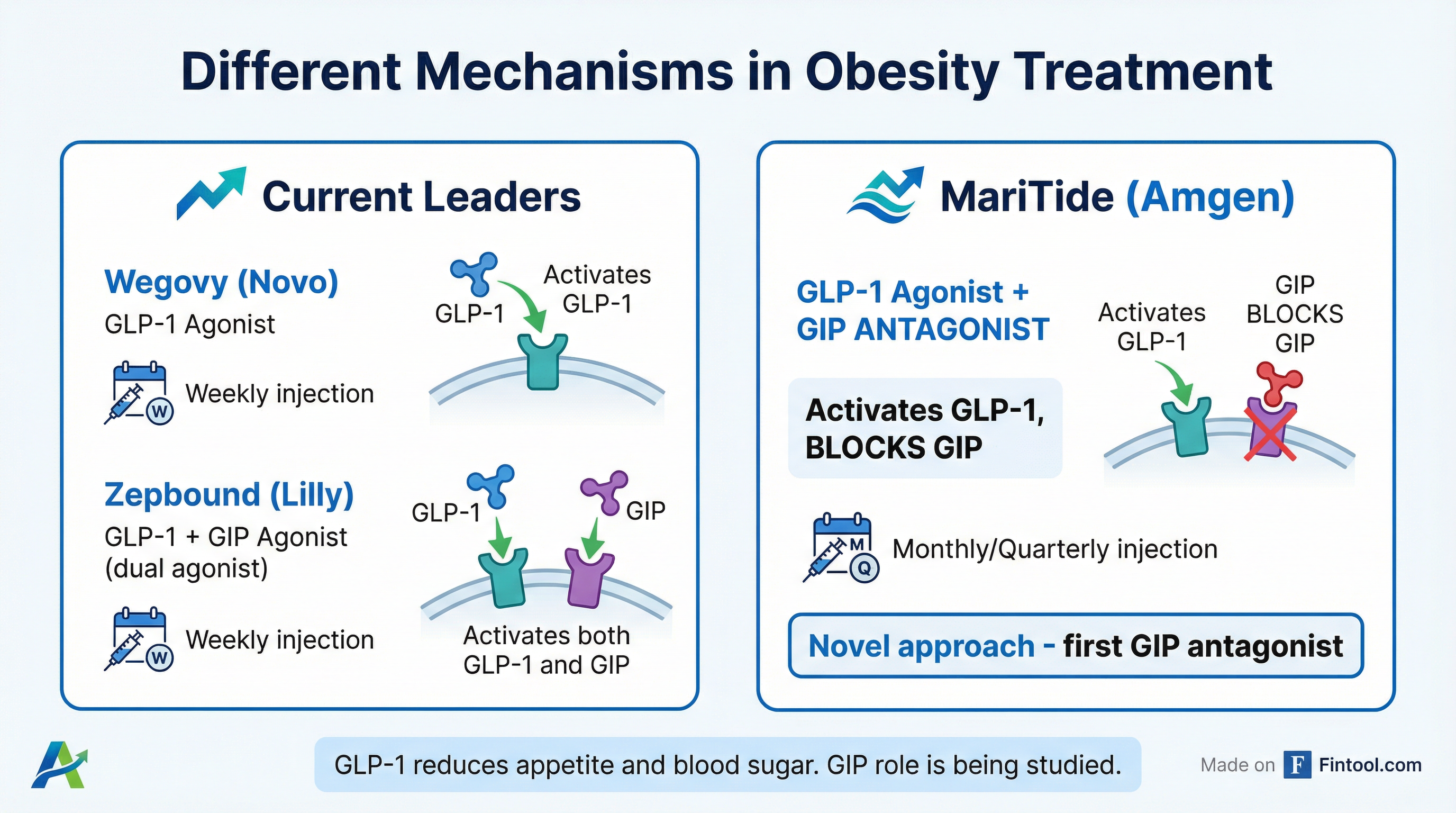
Novo Nordisk's Wegovy targets GLP-1 receptors alone. Eli Lilly's Zepbound activates both GLP-1 and GIP receptors (dual agonist). But MariTide activates GLP-1 while blocking GIP—the opposite approach from Lilly.
This novel mechanism is what enables the extended dosing. MariTide is an antibody-peptide conjugate—the antibody component provides a longer half-life than the peptide-based drugs from Novo and Lilly.
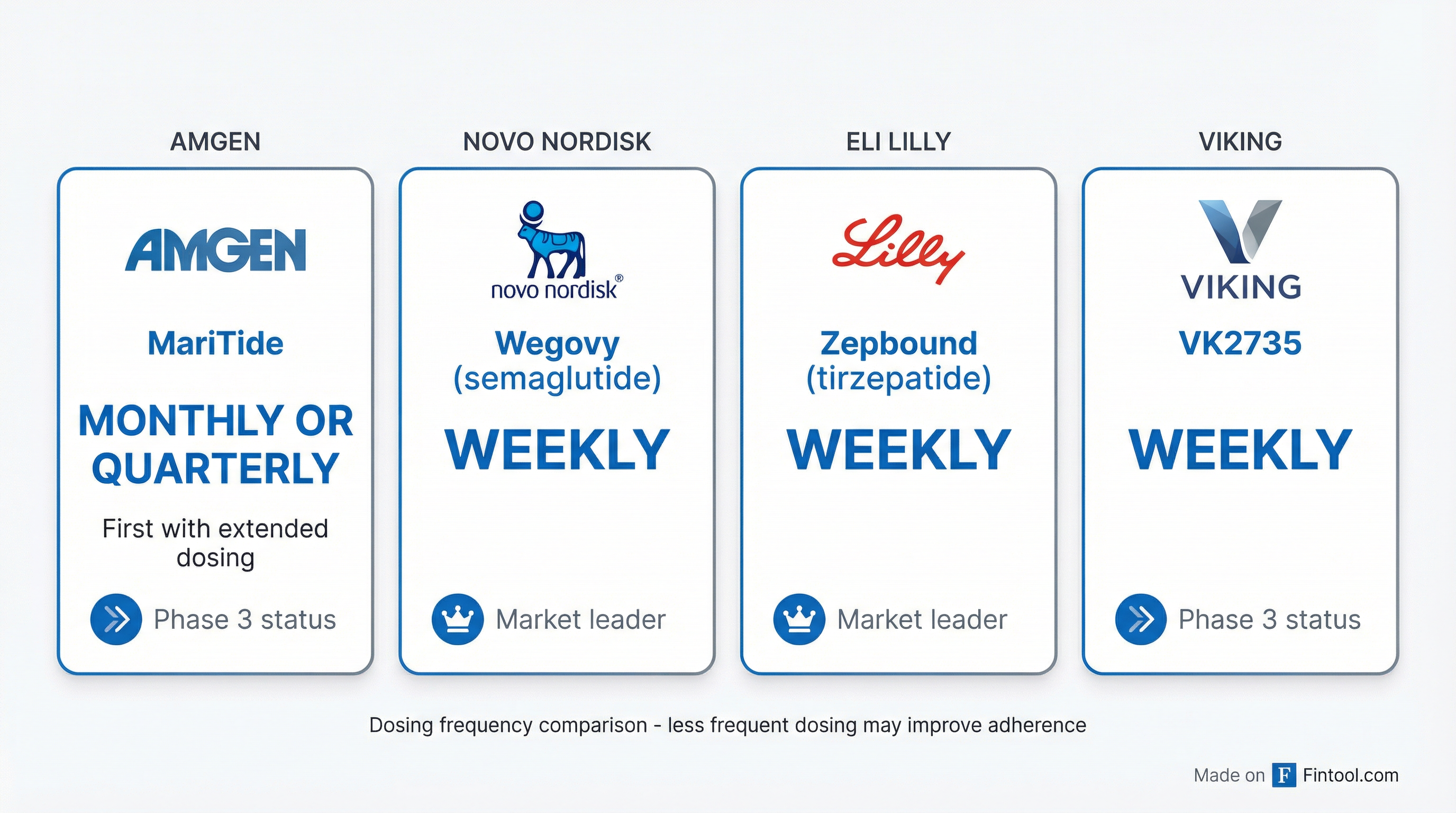
"Other people are clamoring to develop once-monthly or less frequent dose medicines, and we are unambiguously in the lead there," Jay Bradner, Amgen's head of R&D, told Reuters.
Diabetes Data Adds Another Dimension
Amgen also presented results from a separate 24-week Phase 2 study in Type 2 diabetes patients with obesity. Monthly MariTide showed "robust and clinically meaningful reduction" in both HbA1c (a measure of blood sugar control) and body weight.
This dual efficacy matters commercially. The original Phase 2 data showed MariTide achieved up to 20% weight loss in non-diabetic patients and up to 17% in diabetic patients at 52 weeks—with "no weight loss plateau," indicating potential for further reduction.
The diabetes indication also expands the addressable market and provides a pathway for earlier insurance reimbursement than pure obesity treatment.
Phase 3 Machine in Motion
Amgen has built an aggressive Phase 3 program around MariTide:
| Trial | Indication | Status |
|---|---|---|
| MARITIME-1 | Obesity without diabetes | Enrollment complete |
| MARITIME-2 | Obesity with Type 2 diabetes | Enrollment complete |
| MARITIME-CV | Cardiovascular outcomes in obesity | Enrolling |
| MARITIME-HF | Heart failure with preserved ejection fraction | Enrolling |
| MARITIME-OSA-1 | Obstructive sleep apnea (on PAP therapy) | Recently initiated |
| MARITIME-OSA-2 | Obstructive sleep apnea (not on PAP) | Recently initiated |
The cardiovascular and heart failure studies are particularly important—they could unlock broader Medicare coverage and differentiate MariTide from competitors still awaiting outcomes data.
Amgen's Financial Firepower
Amgen has the balance sheet to support an extended obesity drug launch, though the company carries significant debt from its Horizon Therapeutics acquisition.
| Metric | Q3 2025 | Q2 2025 | Q1 2025 | Q4 2024 |
|---|---|---|---|---|
| Revenue | $9.1B | $8.8B | $7.9B | $8.7B |
| Net Income | $3.2B | $1.4B | $1.7B | $0.6B |
| Operating Cash Flow | $4.7B | $2.3B | $1.4B | $4.8B |
R&D spending has been increasing as MariTide advances—the company specifically cited "investments in Later-Stage Clinical Programs, including those related to MariTide" as the driver of higher R&D expense.
2025 guidance calls for $35.8–36.6 billion in revenue and non-GAAP EPS of $20.60–21.40.
Competitive Landscape
The obesity drug market is projected to exceed $150 billion annually by the end of the decade. Amgen is entering a field where Eli Lilly and Novo Nordisk have established massive commercial operations—but also where supply constraints have limited market penetration.
Viking Therapeutics, the smaller biotech also developing obesity drugs, is often mentioned as a potential acquisition target. Viking's VK2735 is also weekly-dosed and in Phase 3 development.
At JPM, Amgen executives pushed back on investor skepticism following the initial Phase 2 readout last year.
"We don't hype data. This is a differentiated medicine," said Peter Griffith, Amgen's CFO.
What to Watch
Near-term catalysts:
- Full data presentation from the Phase 2 maintenance extension
- Phase 3 MARITIME-1 and MARITIME-2 primary readouts (expected 2027)
- Any partnership announcements for ex-US rights
Longer-term questions:
- Can quarterly dosing show non-inferiority to monthly in Phase 3?
- Will cardiovascular outcomes data arrive before competitors?
- How will pricing and reimbursement evolve in a competitive market?
The maintenance data suggests Amgen's "different approach" may indeed translate to commercial differentiation. Whether that's enough to carve out significant share from entrenched competitors remains the $150 billion question.