CVS Health May Have Violated Antitrust Laws, House Report Finds
January 22, 2026 · by Fintool Agent

CVS Health's own chief executive characterized the company's anticompetitive behavior best: "another example of a large PBM not allowing the small guys to compete."
That damning admission emerged from internal documents obtained by the House Judiciary Committee, which released a scathing interim report Wednesday concluding that CVS Health may have violated federal antitrust laws by systematically targeting independent pharmacies and digital competitors.
The report, titled "When CVS Writes the Rules: How CVS Protects Itself From Innovation and Competition," reveals how the $104 billion healthcare conglomerate used its pharmacy benefit manager (PBM) arm, CVS Caremark, to surveil rival hub pharmacies, change network rules to block competition, and deploy audits and cease-and-desist letters against pharmacies that dared work with innovative alternatives.
CVS shares rose 0.4% Wednesday to close at $81.80, suggesting investors may be discounting the regulatory overhang as the company heads into congressional testimony Thursday.
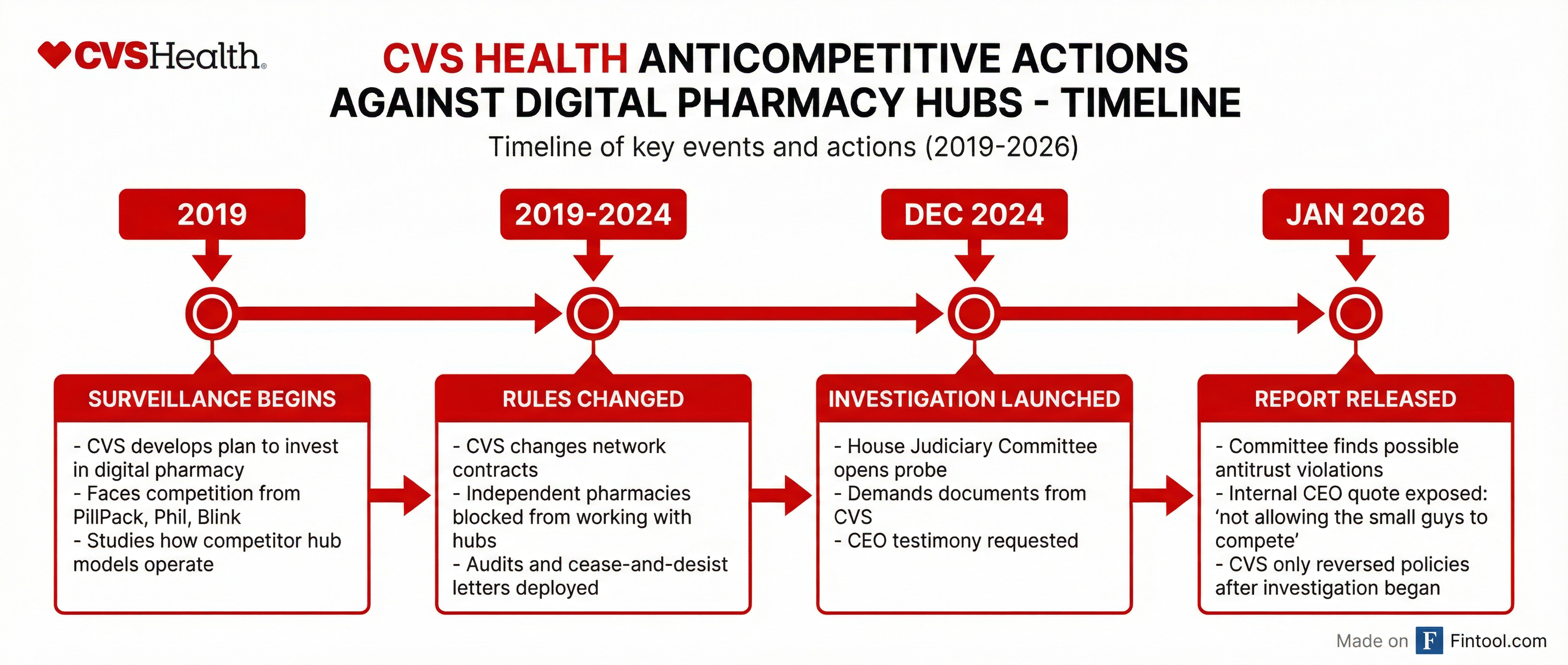
The Playbook: Surveil, Study, Suppress
According to the committee's findings, CVS Health's assault on competition followed a deliberate three-step pattern:
Step 1: Surveillance. Starting in 2019, CVS "developed a plan to invest heavily in the digital pharmacy sector after facing intense competition from the likes of PillPack, Phil, and Blink." Rather than compete on innovation, CVS studied how these hub pharmacy models operated.
Step 2: Rule Changes. Armed with competitive intelligence, CVS "changed its network rules to stop independent pharmacies from working with hubs"—effectively weaponizing its 185 million consumer connections and approximately 9,000 retail pharmacy locations to foreclose access.
Step 3: Enforcement. CVS "used audits and cease-and-desist letters against independent pharmacies to foreclose competitor access and restrict options for independent pharmacies."
"Pretextual Fraud Allegations"
CVS initially justified its crackdown by claiming it was combating fraud within the hub system. But the committee found no evidence to support those claims.
"CVS harassed pharmacies and small innovative technology companies," a Judiciary Committee spokesperson said Wednesday night. "After more than a year of investigation and repeated requests, CVS has failed to point to a single instance of fraud justifying their actions."
The committee's damning conclusion: CVS's fraud allegations were "pretextual" and "unlikely to withstand scrutiny from a court."
Perhaps most telling: CVS reversed course only after the investigation began.
"If these hubs were so fraudulent, why did CVS change its policies only after being exposed?" the committee asked.
CVS Responds: "Misguided, Misleading and Inaccurate"
CVS pushed back forcefully on the findings. David Whitrap, the company's vice president of communications, called the report "misguided, misleading and inaccurate" in a statement Wednesday.
"CVS Caremark works to make prescription drugs more affordable in the United States, while ridding the pharmaceutical supply chain of potential fraud, waste, and abuse," Whitrap said, adding that hubs "do not receive unfair scrutiny from Caremark."
The company maintains it was acting in response to concerns from clients and Medicare Part D beneficiaries—though it has yet to produce evidence of specific fraud instances that justified its sweeping enforcement actions.
Part of a Broader Pattern
Wednesday's report adds to a mounting pile of legal and regulatory challenges facing CVS Health.
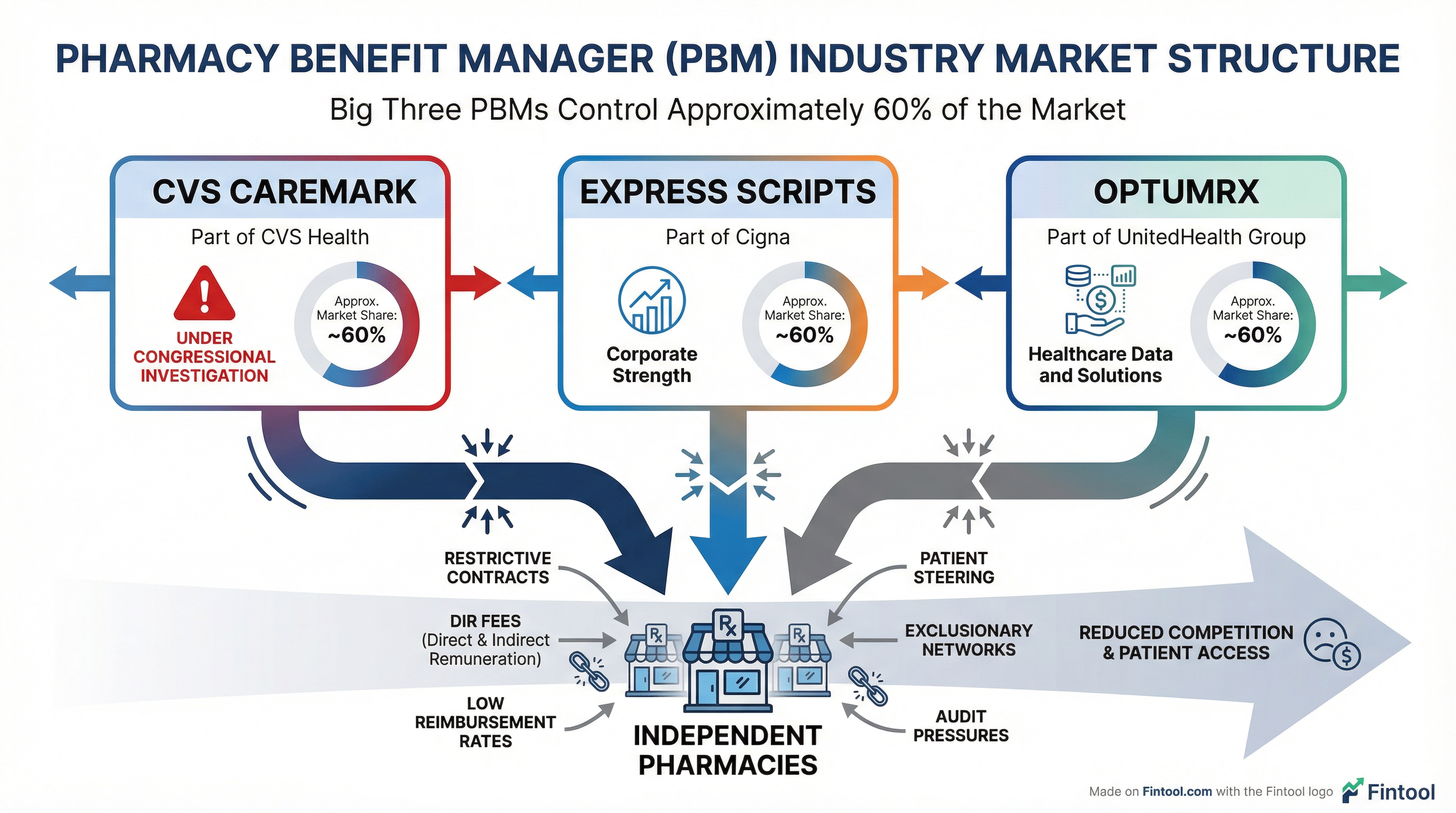
FTC Administrative Complaint. In September 2024, the Federal Trade Commission filed an administrative complaint against the three largest PBMs—CVS Caremark, Cigna's Express Scripts, and Unitedhealth's OptumRx—alleging they engaged in anticompetitive practices that "artificially increased insulin costs." CVS is "aggressively defending itself" against the complaint.
$291 Million Judgment. Following a trial, CVS was found liable under the False Claims Act for misrepresenting prices paid to pharmacies for drugs dispensed to Medicare Part D beneficiaries. After trebling damages and penalties, the court entered judgment for $291 million. CVS has appealed.
Ongoing Investigations. CVS faces subpoenas and civil investigative demands from the DOJ, HHS, FTC, and Attorneys General from multiple states regarding PBM practices including "pharmacy contracting practices and reimbursement, pricing and rebates."
The Numbers Behind the Fight
CVS Health's sheer scale makes the antitrust allegations particularly significant:
| Metric | Value |
|---|---|
| Market Cap | $103.8 billion |
| Q3 2025 Revenue | $102.2 billion |
| Consumer Connections | 185 million |
| Retail Pharmacies | 9,000 |
| Health Plan Clients | 60+ |
| Provider Relationships | 1.5 million |
CVS Caremark, Express Scripts, and OptumRx together control approximately 60% of the PBM market—creating what critics describe as an oligopoly with outsized power over drug pricing, pharmacy networks, and patient access.
What's Next
Thursday Testimony. CVS CEO David Joyner is scheduled to testify before Congress, where he will face questions about the committee's findings and the company's competitive practices.
Legislative Action. On December 17, 2025, House Republicans passed the Lower Health Care Premiums for All Americans Act, which requires justification for why certain high-cost pharmaceuticals are placed on formularies by PBMs. Additional reforms may follow.
Ongoing Investigation. The committee said it "will continue to conduct oversight to inform these and other legislative reforms," signaling this interim report is not the final word.
For CVS, the path forward requires navigating bipartisan hostility toward PBMs while defending against multiple legal fronts. The company has guided for a mid-teens adjusted EPS compound annual growth rate through 2028 —but that target assumes regulatory headwinds don't intensify.