Eli Lilly Eyes €15 Billion Abivax Takeover as Pharma M&A Heats Up
January 12, 2026 · by Fintool Agent

Abivax+6.29% shares surged as much as 30% to a record high Monday after French publication La Lettre reported that Eli Lilly+3.66% is preparing a €15 billion ($17.5 billion) takeover bid for the Paris-based biotech—potentially the pharma industry's largest deal of 2026.
The rumored offer, which values Abivax at nearly double its current market capitalization, would give Lilly access to obefazimod, a first-in-class oral drug for inflammatory bowel disease that CEO Marc de Garidel says could become "one of the most-used products in the next decade."
No formal bid has been submitted. Lilly is reportedly seeking guidance from the French Finance Ministry on whether the acquisition would trigger foreign investment controls—a review that typically takes up to three months.

The Prize: A Potential Blockbuster in IBD
Obefazimod is the first drug to target microRNA-124 (miR-124), a natural regulator of inflammation. Unlike biologics that suppress the immune system, it works by stabilizing the body's own anti-inflammatory mechanisms—a potentially safer approach for long-term treatment of chronic conditions like ulcerative colitis and Crohn's disease.
The Phase 3 ABTECT trials, reported in July 2025, demonstrated strong efficacy:
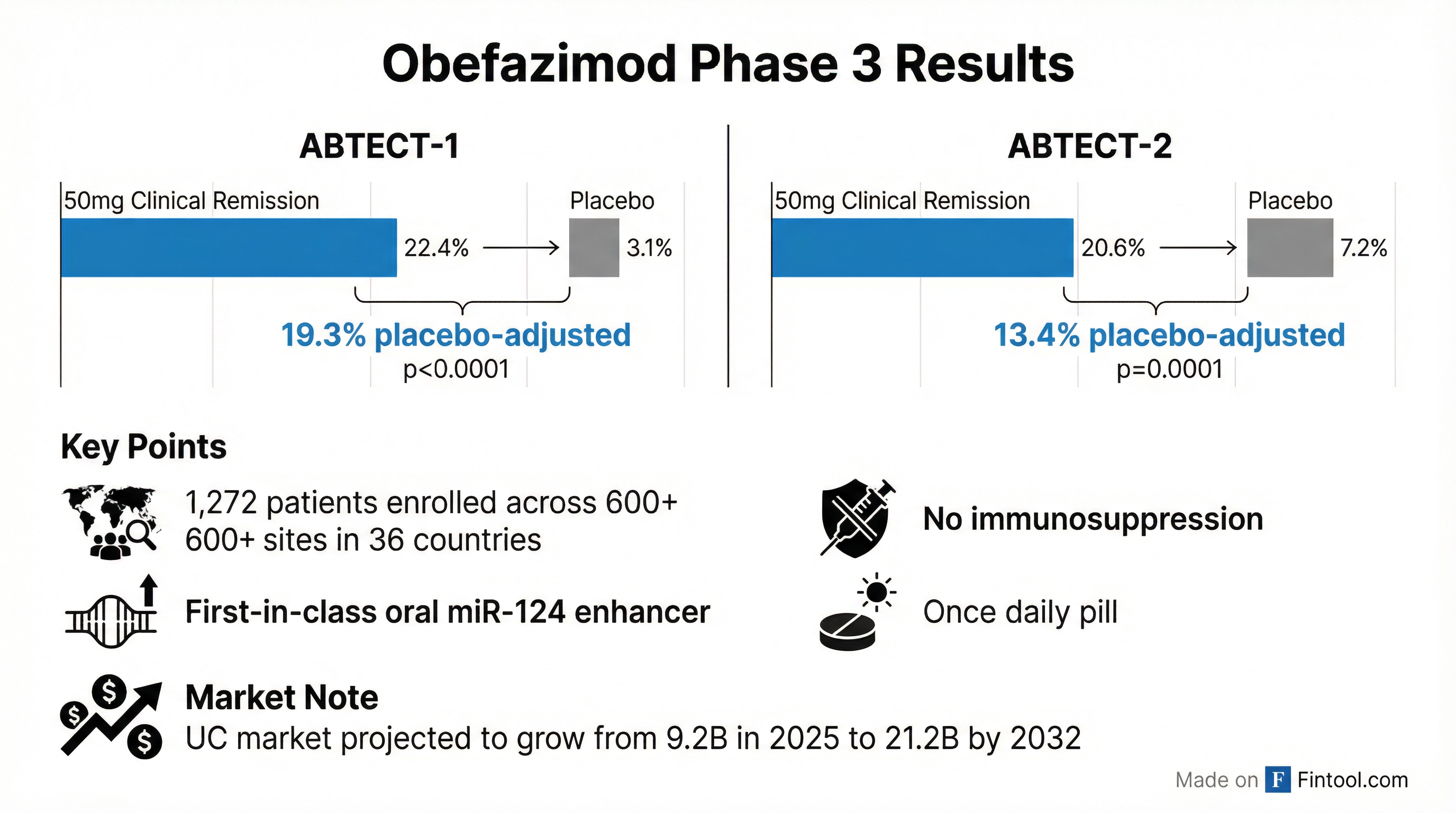
| Trial | 50mg Clinical Remission | Placebo | Placebo-Adjusted | P-Value |
|---|---|---|---|---|
| ABTECT-1 | 22.4% | 3.1% | 19.3% | <0.0001 |
| ABTECT-2 | 20.6% | 7.2% | 13.4% | 0.0001 |
The ABTECT program enrolled 1,272 patients across 600+ clinical trial sites in 36 countries—one of the largest Phase 3 ulcerative colitis trials ever conducted.
"The exciting thing is that obefazimod is oral. It's once daily, so it's convenient. It's a small molecule, so it won't be immunogenic as some biologics can be," said Dr. Bruce Sands, Chief of Gastroenterology at Mount Sinai, who presented the Phase 3 data.
Market Opportunity: UC Sales Could Double by 2032
The timing of Lilly's interest reflects the explosive growth expected in the inflammatory bowel disease market. Third-party analyses forecast the ulcerative colitis market will more than double from $9.2 billion in 2025 to $21.2 billion by 2032, driven by increased adoption of advanced therapies and new mechanisms of action.
Market research following the ABTECT Phase 3 results indicates obefazimod could emerge as the future market leader in UC, potentially outperforming both currently approved therapies and anticipated future entrants including TL1A inhibitors and oral IL-23 agents.
Abivax estimates roughly 500,000 US patients are currently on conventional UC therapies, with approximately 60% (300,000 patients) having moderate-to-severe disease—prime candidates for advanced therapies like obefazimod.
Lilly's M&A Spree Continues
The potential Abivax deal would mark the capstone of an aggressive M&A campaign that has seen Lilly deploy over $10 billion on acquisitions since 2024—all while generating record revenue from its GLP-1 obesity and diabetes franchise.
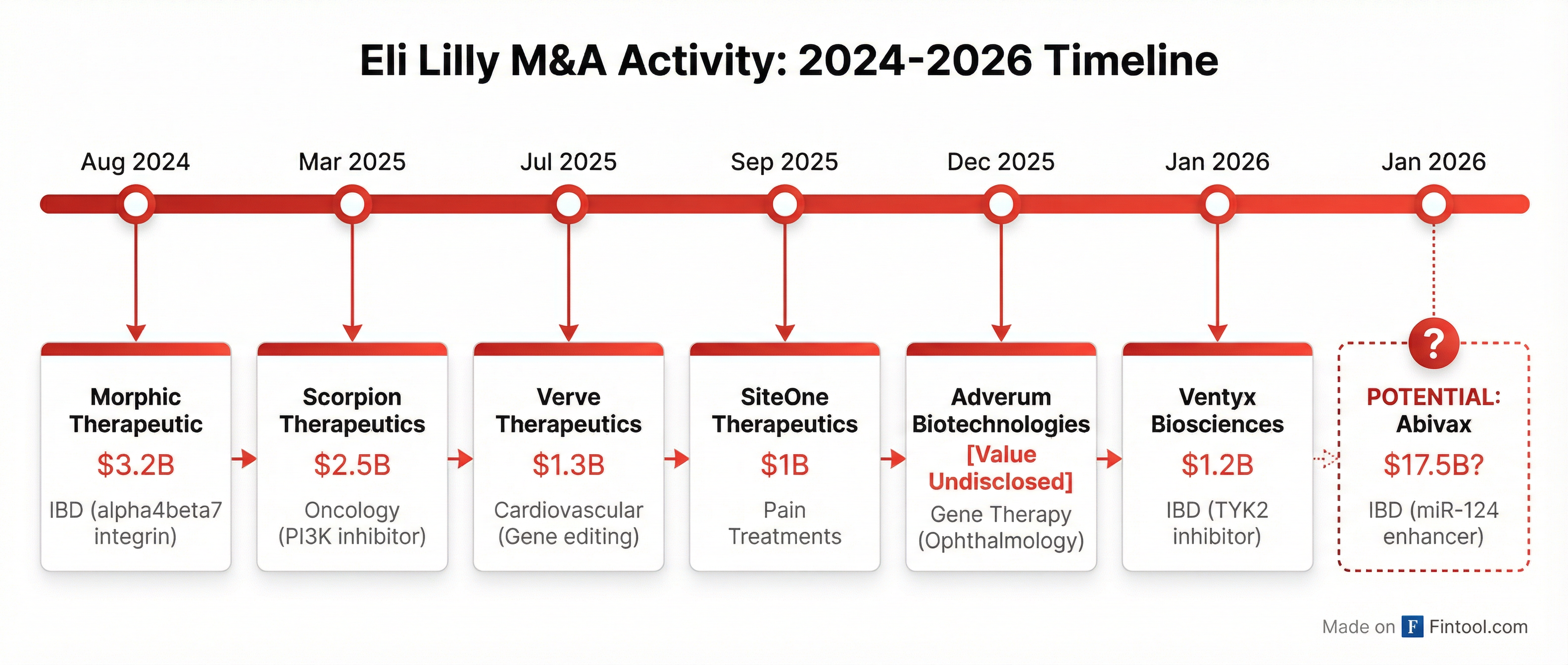
| Date | Target | Value | Therapeutic Area |
|---|---|---|---|
| Aug 2024 | Morphic | $3.2B | IBD (α4β7 integrin) |
| Mar 2025 | Scorpion | $2.5B | Oncology (PI3Kα) |
| Jul 2025 | Verve | $1.3B | Cardiovascular (gene editing) |
| Sep 2025 | SiteOne | $1.0B | Pain (non-opioid) |
| Dec 2025 | Adverum | - | Ophthalmology (gene therapy) |
| Jan 2026 | Ventyx | $1.2B | IBD (TYK2 inhibitor) |
| Jan 2026 | Abivax? | $17.5B? | IBD (miR-124) |
Just five days ago, Lilly announced its $1.2 billion acquisition of Ventyx Biosciences, which brought another IBD-focused oral therapy pipeline—this time targeting TYK2 inhibition.
Lilly already has an established IBD presence through Omvoh (mirikizumab), which received approval for Crohn's disease in the US, EU, and Japan in 2025. Adding obefazimod would give Lilly a complementary oral option with a differentiated mechanism—and potentially the most comprehensive IBD portfolio in the industry.
Lilly's Financial Firepower
Eli Lilly has the balance sheet to absorb a $17.5 billion acquisition. The company reported Q3 2025 revenue of $17.6 billion (up 45% year-over-year) and net income of $5.6 billion, driven by explosive growth in Mounjaro and Zepbound.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $13.5B | $12.7B | $15.6B | $17.6B |
| Net Income | $4.4B | $2.8B | $5.7B | $5.6B |
| Cash | $3.3B | $3.1B | $3.4B | $9.8B |
The company's cash position nearly tripled in Q3 to $9.8 billion, providing significant liquidity. With a market cap of approximately $950 billion and strong cash generation, Lilly could easily finance the deal through a combination of cash and debt.
The French Regulatory Question
The key near-term uncertainty is French foreign investment controls. France has become increasingly protective of domestic biotech assets, and a €15 billion acquisition by an American pharma giant would almost certainly trigger regulatory review.
Abivax CEO Marc de Garidel told Bloomberg that a Treasury review is "necessary in France and typically takes up to three months." He declined to comment on the Lilly rumor specifically, but emphasized that the company would need a partner to bring obefazimod to international markets.
"If you are Big Pharma, you cannot ignore that this product may be one of the most-used products in the next decade," de Garidel said.
JPMorgan Healthcare Conference Context
The timing of the Abivax rumor coincides with the JPMorgan Healthcare Conference in San Francisco, which kicks off Monday—historically the most active week for pharma M&A announcements.
2026 has already started hot for pharma deals:
- Amgen completed an $840 million acquisition of Dark Blue Therapeutics
- Eli Lilly announced its $1.2 billion Ventyx deal
- Merck is rumored to be in talks to buy Revolution Medicines for up to $32 billion
Analyst Mehdi Telliez at Kepler Cheuvreux raised his target price on Abivax to the highest level tracked by Bloomberg, noting that a takeover "could reasonably clear in a €150–250 per share range" with a control premium.
What to Watch
Q2 2026: ABTECT Maintenance Trial Results Topline data from the 44-week maintenance study will be critical. The December 2025 DSMB meeting found no new safety signals with over 80% of participants completing the double-blind maintenance phase.
Late 2026: FDA Filing Abivax plans to submit a New Drug Application pending successful maintenance results. If Lilly acquires the company, it would likely accelerate the regulatory and commercial launch timeline.
French Regulatory Review Watch for Finance Ministry guidance on foreign investment controls. Approval—or rejection—would signal whether Lilly can proceed with a formal bid.
Competitive Bids At €15 billion, other pharma giants may enter the fray. Abbvie+2.01%, Johnson & Johnson+0.93%, and Merck+1.82% all have significant IBD franchises and could see Abivax as strategically valuable.