Lilly Stock Drops 4% as FDA Delays Obesity Pill, Handing Novo Crucial Head Start
January 15, 2026 · by Fintool Agent

Eli Lilly+3.66% shares tumbled nearly 4% Thursday after the FDA extended its review of orforglipron—the company's highly anticipated oral obesity pill—to April 10, 2026, handing rival Novo Nordisk+9.92% a crucial three-month head start in what could become a $100 billion market.
The delay wiped approximately $35 billion from Lilly's market capitalization, sending shares from $1,073 to around $1,031 as investors recalibrated expectations for the drugmaker's next major growth catalyst.
The Oral GLP-1 Race Just Got More Lopsided
The timing couldn't be worse for Lilly. Just ten days ago, Novo Nordisk launched oral Wegovy nationwide—the first oral GLP-1 approved for weight loss—at a starting price of $149 per month.
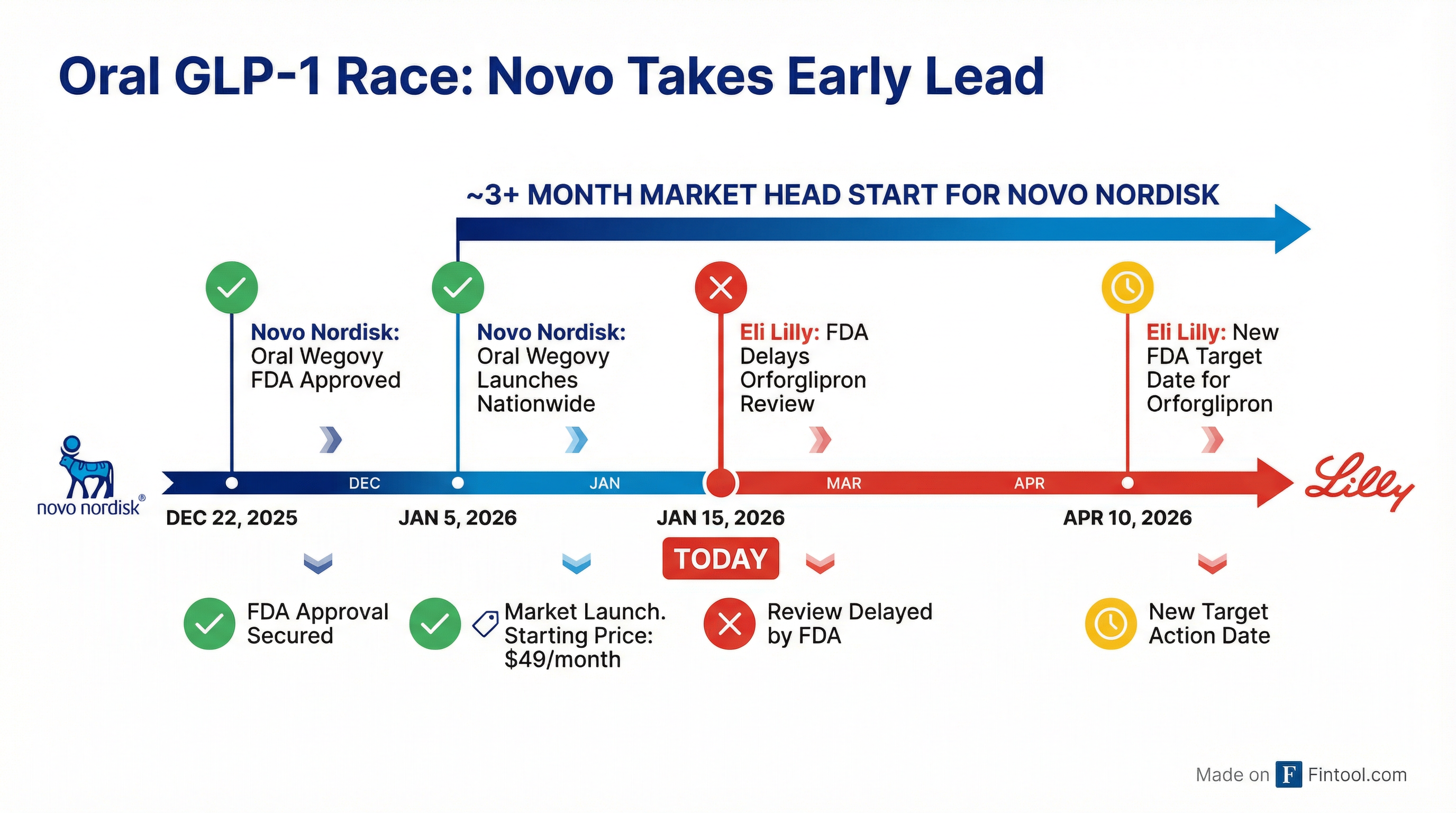
The FDA's original target date for orforglipron was March 28, and Lilly CEO David Ricks had previously signaled the drug could reach the market as early as that month. Now, even with approval on April 10, Novo will have enjoyed at least three months of market exclusivity in the oral obesity space.
"The launch of Wegovy pill in 2021 changed how obesity was viewed and treated in the US," Novo's Dave Moore said at the January 5 launch. "Now, with Wegovy pill, we are offering a magnitude of weight loss that no other oral GLP-1 obesity candidate has been able to duplicate in Phase III trials."
Priority Voucher Program Hits Turbulence
The orforglipron delay is part of a broader pattern emerging in the FDA's new Commissioner's National Priority Voucher program, which promised approval decisions in just 1-2 months for drugs deemed critical to public health or national security.
The FDA also extended reviews for three other priority voucher drugs on the same day:
| Drug | Company | Original Timeline | New Target | Reason |
|---|---|---|---|---|
| Orforglipron | Eli Lilly | March 28 | April 10 | Not disclosed |
| Tzield | Sanofi | — | +1 month | Safety signals (2 seizures, 1 death) |
| Bitopertin | Disc Medicine | — | +2 weeks | Efficacy concerns, abuse risk |
| Zongertinib | Boehringer | — | Mid-February | Not disclosed |
The delays raise questions about whether the accelerated program—launched in June 2025 under the Trump administration—can deliver on its promise of dramatically shortened review timelines without compromising safety standards.
Lilly's Billions Bet on Orforglipron
Eli Lilly+3.66% has committed over $20 billion in manufacturing investments specifically targeting orforglipron production capacity, signaling the company's conviction that oral GLP-1s will dominate the next phase of the obesity market.
Recent Orforglipron Manufacturing Announcements:
- Huntsville, Alabama (Dec 2025): $6 billion facility, 3,450 jobs, completion by 2032
- Houston, Texas (Sept 2025): $6.5 billion facility, 4,615 jobs, operational within 5 years
- Puerto Rico Expansion (Oct 2025): $1.2 billion upgrade, production starting 2028
- Netherlands Site (Nov 2025): $3 billion facility, 500 manufacturing jobs
The company filed its new drug application for orforglipron in late 2025 following four positive Phase 3 trials across type 2 diabetes and obesity indications.
The Financial Stakes
Lilly's incretin portfolio—led by injectable Mounjaro and Zepbound—has already transformed the company into one of pharma's fastest growers. Q3 2025 revenue surged 54% year-over-year to $17.6 billion, driven almost entirely by volume growth from its GLP-1 drugs.
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $13.5B | $12.7B | $15.6B | $17.6B |
| Net Income | $4.4B | $2.8B | $5.7B | $5.6B |
| Gross Margin | 82.2% | 82.5% | 84.3% | 82.9% |
Orforglipron represents Lilly's best opportunity to expand beyond injectable patients. The company has already secured a Medicare pricing deal that would cap out-of-pocket costs at $50/month for beneficiaries once approved, opening access to nearly 40 million Americans on government insurance.
What to Watch
Near-term catalysts:
- April 10, 2026: New FDA target date for orforglipron decision
- Q1 2026 earnings: Management commentary on regulatory timeline and launch readiness
- Novo Nordisk market share data: Early oral Wegovy adoption metrics
Key questions for investors:
- Will the FDA meet the April 10 deadline, or could further delays materialize?
- How much market share can Novo capture in the three-month window?
- Does the priority voucher program's stumbles signal broader regulatory risk?
The delay doesn't change orforglipron's clinical profile—Phase 3 data showed patients maintained weight loss after switching from injectable GLP-1s, with safety consistent across trials. But in a market where first-mover advantage can determine long-term positioning, every week of delay hands Novo Nordisk another head start.
Related: