Eli Lilly Nears $1B+ Ventyx Deal as TYK2 Arms Race Heats Up
January 7, 2026 · by Fintool Agent

Ventyx Biosciences+0.14% shares exploded more than 60% after hours Tuesday following reports that Eli Lilly+3.66% is in advanced talks to acquire the clinical-stage biotech for over $1 billion. The deal, which could be announced imminently, would give Lilly a TYK2 inhibitor for Crohn's disease—adding a third mechanism of action to its rapidly expanding inflammatory bowel disease franchise.
VTYX closed the regular session at $10.05, up 28.5% from Monday's close of $7.82, after touching an intraday high of $25. In after-hours trading, shares surged to $16.22—representing a roughly 107% premium to the pre-news closing price.
The Strategic Rationale: Building an IBD Powerhouse
Lilly's interest in Ventyx is a natural extension of its aggressive push into gastroenterology. In August 2024, Lilly completed its $3.2 billion acquisition of Morphic Holding, gaining MORF-057, an oral α4β7 integrin inhibitor in Phase 2 trials for ulcerative colitis and Crohn's disease.
Combined with Omvoh (mirikizumab), Lilly's approved IL-23p19 antibody for UC and Crohn's, a Ventyx acquisition would give the Indianapolis pharma giant three distinct mechanisms to attack IBD:
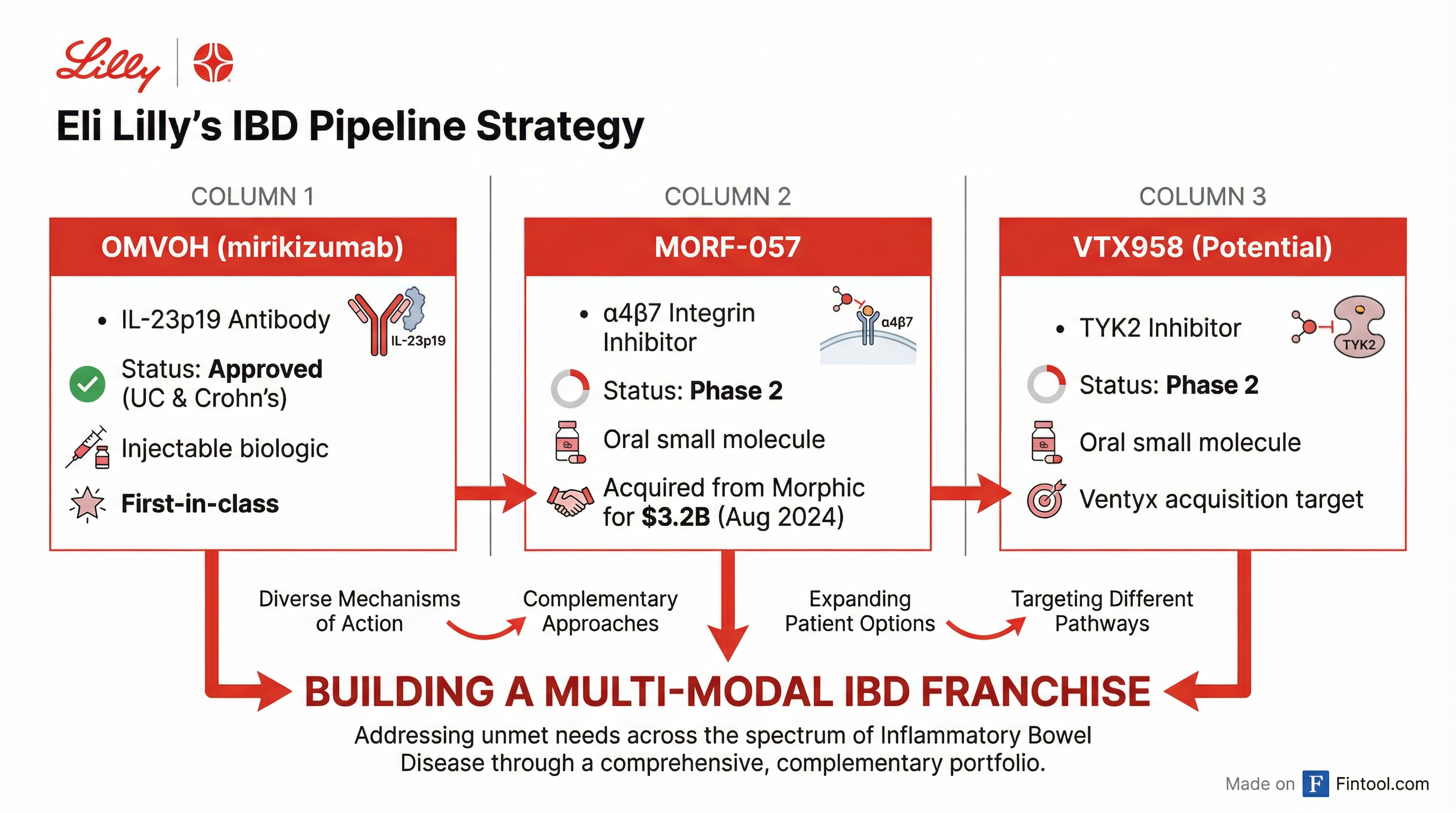
"Acquiring Morphic reinforces our growing capabilities in gastroenterology, building on the strong foundation of Omvoh," said Daniel Skovronsky, Lilly's chief scientific officer, when that deal closed. "The acquisition allows Lilly to research potential combination treatments that could better serve people beyond what is possible with currently available medicines."
The same logic applies to Ventyx's VTX958, an oral TYK2 inhibitor with Phase 2 data suggesting potential disease-modifying benefits in Crohn's disease.
What Lilly Gets: A Diversified Pipeline
While VTX958 is likely the crown jewel, Ventyx brings more to the table:
| Asset | Mechanism | Indication | Status | Key Data |
|---|---|---|---|---|
| VTX958 | TYK2 Inhibitor | Crohn's Disease | Phase 2 | Dose-dependent endoscopic response, CRP/calprotectin reduction |
| VTX3232 | CNS-penetrant NLRP3 Inhibitor | Cardiovascular/Parkinson's | Phase 2 | 80% hsCRP reduction; significant IL-6, Lp(a), fibrinogen reductions |
| VTX2735 | Peripherally-restricted NLRP3 Inhibitor | Recurrent Pericarditis | Phase 2 | Data expected Q4 2025 |
| Tamuzimod | S1P1R Modulator | Ulcerative Colitis | Phase 2 | Robust clinical/endoscopic remission rates vs. placebo |
The cardiovascular angle is particularly intriguing. Ventyx's Phase 2 data showed VTX3232, when combined with semaglutide, demonstrated significant reductions in inflammatory markers relative to semaglutide alone—a potential "GLP-1 plus" strategy that could appeal to Lilly's obesity franchise ambitions.
TYK2: The Hottest Space in Immunology
The timing of this deal coincides with a flurry of activity in the TYK2 inhibitor market. Just yesterday, Alumis reported positive Phase 3 data for its TYK2 inhibitor envudeucitinib in psoriasis, sending its shares up 95%. Takeda is also planning regulatory filings for zasocitinib in 2026.
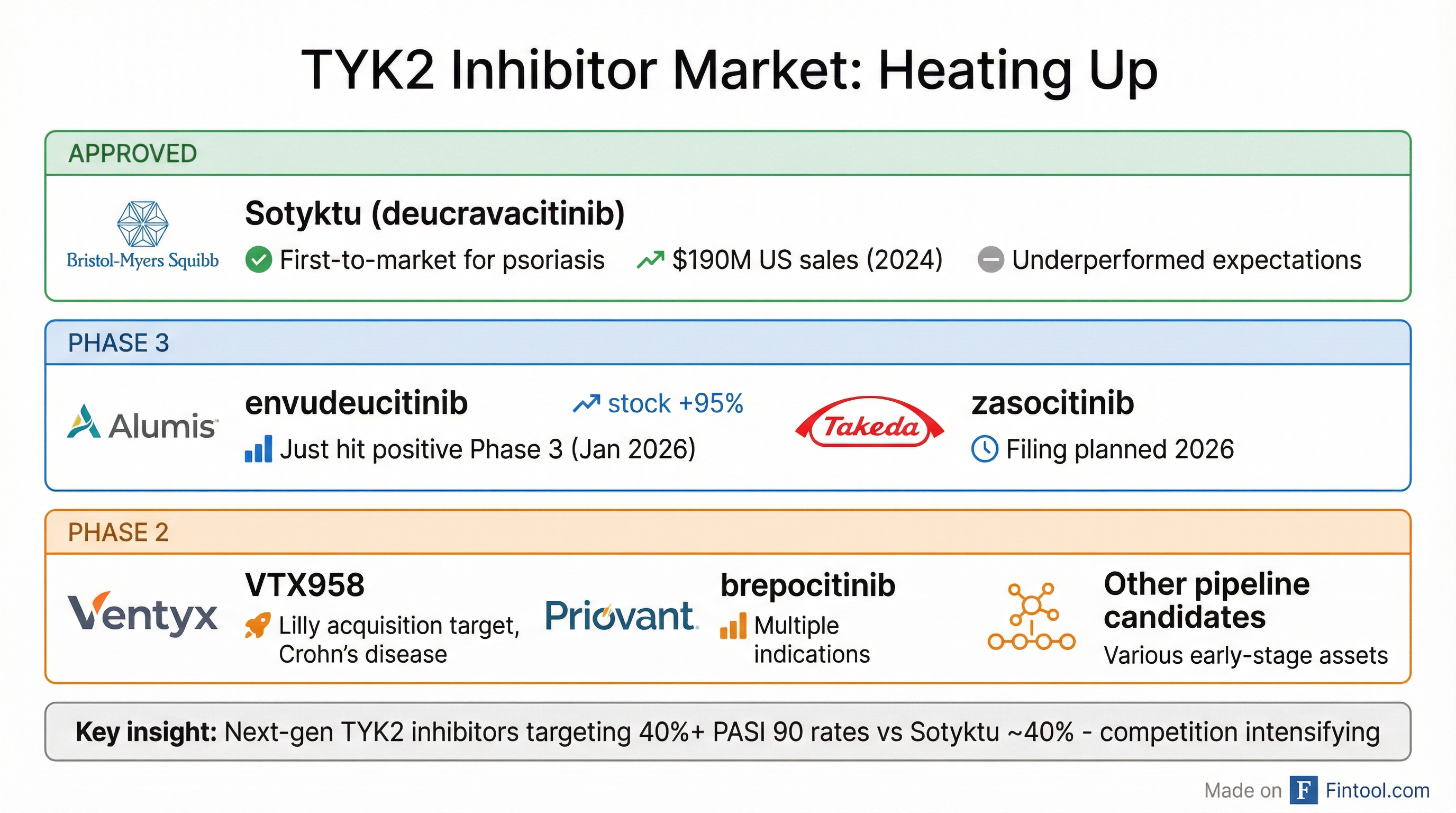
The market leader, Bristol-myers Squibb's+4.15% Sotyktu (deucravacitinib), has underperformed expectations since its 2022 launch, generating $190 million in US sales in 2024—a 32% increase, but well below the blockbuster trajectory BMS had projected.
The knock on Sotyktu: efficacy that falls short of injectable biologics like Novartis's Cosentyx and AbbVie's Skyrizi. Next-generation TYK2 inhibitors like Alumis's envudeucitinib are targeting higher skin clearance rates, potentially reshaping the competitive dynamics.
Deal Economics: Premium to a Beaten-Down Stock
Ventyx's stock had been on a wild ride. From a 52-week low of $0.78, shares rallied to nearly $9 in late 2025 as positive clinical data emerged from multiple programs. The reported >$1 billion valuation implies roughly $14+ per share—a significant premium to the $7.82 pre-news close, though below the $25 intraday high touched during Tuesday's session.
| Metric | Value |
|---|---|
| Pre-news close (Jan 5) | $7.82 |
| Regular close (Jan 6) | $10.05 |
| Intraday high (Jan 6) | $25.00 |
| After-hours price | $16.22 |
| 52-week range | $0.78 - $25.00 |
| Shares outstanding | 71.3M |
| Cash position (Q3 2025) | $192.6M |
| Quarterly burn rate | $23M |
Ventyx had been running lean, with Q3 2025 R&D expenses of $17.7 million versus $30.6 million a year earlier. The company was actively exploring partnership opportunities for VTX958 and tamuzimod—signals that management recognized the need for external capital or a strategic exit.
Lilly's M&A Playbook
This potential deal follows Lilly's established playbook of acquiring clinical-stage assets with differentiated mechanisms:
| Date | Target | Value | Key Asset | Therapeutic Area |
|---|---|---|---|---|
| Aug 2024 | Morphic | $3.2B | MORF-057 | IBD (α4β7 integrin) |
| Mar 2025 | Scorpion | $1.4B | STX-478 | Oncology (PI3Kα) |
| Jul 2025 | Verve | $1.3B | PCSK9 gene editing | Cardiovascular |
| Jan 2026 | Ventyx* | >$1B | VTX958 | IBD (TYK2) |
*Reported, not yet announced
Lilly's balance sheet can easily absorb these deals. The company reported $9.8 billion in cash as of Q3 2025 and generated $5.6 billion in net income during the quarter.
What to Watch
Near-term catalysts:
- Official deal announcement (reportedly imminent)
- Deal structure details—all-cash vs. CVRs
- Regulatory review timeline (likely Hart-Scott-Rodino clearance)
Clinical milestones:
- VTX2735 Phase 2 data in recurrent pericarditis (expected Q4 2025/Q1 2026)
- VTX958 regulatory strategy under Lilly stewardship
- Potential combination studies with Omvoh or MORF-057
Competitive dynamics:
- Alumis Phase 3 readouts and FDA filing timeline
- Takeda zasocitinib regulatory submissions
- Bristol-Myers Squibb's Sotyktu label expansion efforts
The Bottom Line
If this deal closes, Lilly will have spent over $5.5 billion in 18 months building what may become the most comprehensive IBD franchise in the industry. The combination of an approved biologic (Omvoh), an oral integrin inhibitor (MORF-057), and potentially an oral TYK2 inhibitor (VTX958) positions Lilly to offer physicians multiple options—and potentially combinations—for patients with treatment-refractory disease.
For Ventyx shareholders, the premium to the pre-news price represents a strong exit for a company that was actively seeking partners. For Lilly, it's another bet that differentiated mechanisms in immunology will pay off as the market shifts toward oral therapies and away from injectable biologics.