Merck Beats Q4 Estimates But Guides Low as Gardasil Headwinds Persist
February 3, 2026 · by Fintool Agent

Merck delivered a mixed Q4 earnings report Tuesday morning, beating both EPS and revenue estimates while simultaneously issuing 2026 guidance that fell short of Wall Street expectations. The divergence reflects the company's core challenge: a $31.7 billion oncology franchise firing on all cylinders while its vaccine business, particularly the HPV shot Gardasil, continues to struggle with China headwinds and regulatory changes in the U.S.
Fourth-quarter adjusted earnings came in at $2.04 per share versus the $2.01 consensus, while revenue of $16.4 billion topped the $16.2 billion estimate . Yet for full-year 2026, Merck projected sales of $65.5-67 billion versus Street expectations of $67.6 billion, and adjusted EPS of $5.00-5.15 versus the $5.27 consensus .
The stock initially dipped 1.5% at the open to $111.67 but rallied throughout the session, closing at $116.37—up 4.2%—as investors focused on the Q4 beat and pipeline momentum rather than the guidance miss.
The Gardasil Problem
The headline challenge for Merck is Gardasil, the HPV vaccine that generated $8.6 billion in 2024 but collapsed to just $5.2 billion in 2025—a 39% decline .
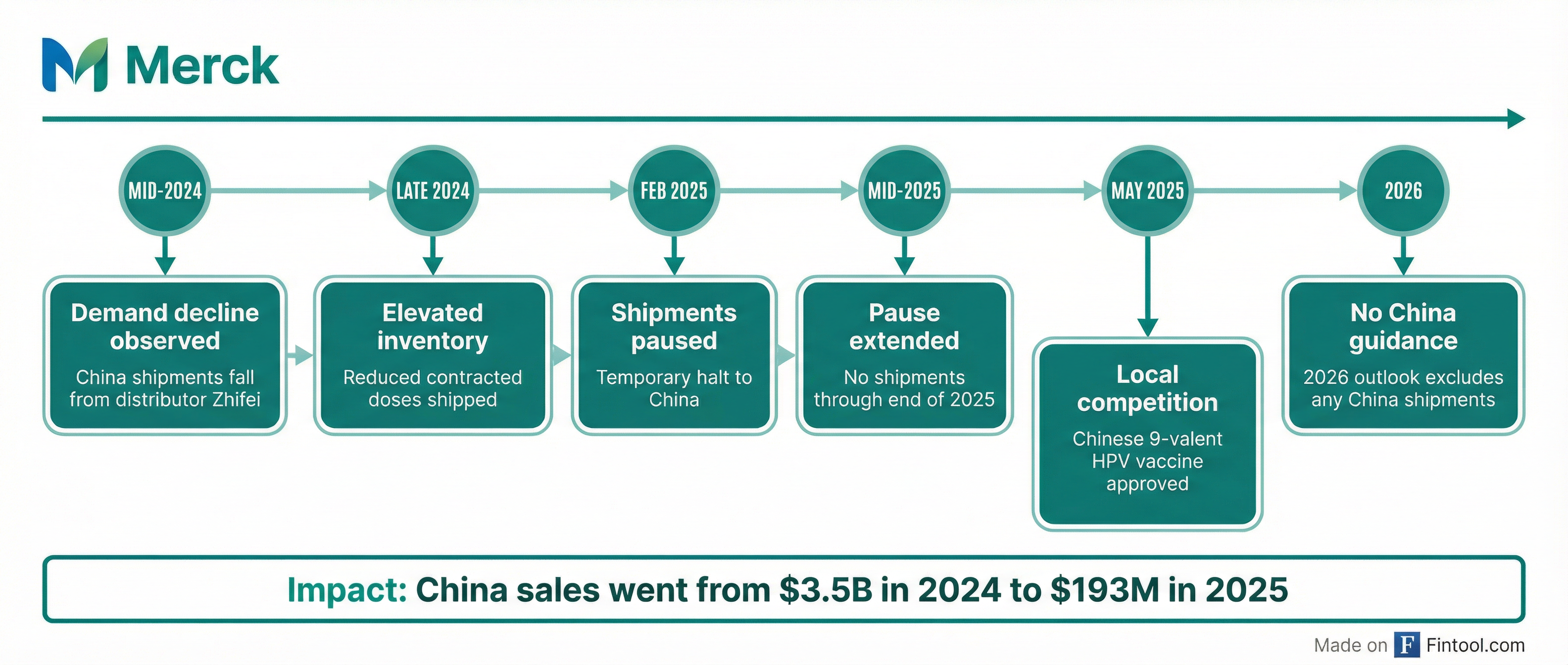
China has been the primary driver. Sales to China dropped from $3.5 billion in 2024 to just $193 million in 2025 after Merck paused shipments in February 2025 due to elevated inventory levels at its distributor, Zhifei . The company confirmed it will not resume shipments through at least the end of 2025, and its 2026 guidance assumes no China Gardasil revenue at all .
Adding to the pressure, a Chinese competitor launched a nine-valent HPV vaccine in May 2025 at lower prices . And in January 2026, the CDC reduced its recommended doses from two-to-three to just one for children—a change Jefferies estimates could cost Merck $315-630 million in annual U.S. revenue.
"Lower demand in China persisted," CFO Caroline Litchfield said on the call. "At the end of 2024, overall channel inventory levels in China remained elevated at above normal levels" .
Keytruda: The $32 Billion Anchor
While Gardasil struggles, Keytruda continues to dominate. The cancer immunotherapy generated $31.7 billion in 2025 sales, up 7% year-over-year, including $40 million from the newly launched subcutaneous formulation, Keytruda QLEX .
Q4 saw Keytruda sales reach $8.4 billion, up 5% despite approximately $200 million in timing headwinds from U.S. wholesaler purchases . Growth was driven by continued uptake in earlier-stage cancers, particularly in breast, cervical, and endometrial indications, as well as the new combination with Padcev for urothelial cancer .
But the Keytruda franchise faces its own cliff. The compound patent expires in December 2028, with method-of-use patents extending to late 2029. CEO Rob Davis noted on the call that case law developments have increased management's confidence in defending the extended patents, but for "planning purposes, we continue to assume 2028 because I think that's the conservative assumption" .
Merck also faces the Inflation Reduction Act's drug price negotiations starting in 2029, creating a double headwind for its flagship product.
| Metric | Q4 2025 | Q4 2024 | Change |
|---|---|---|---|
| Keytruda/QLEX Revenue | $8.4B | $7.8B | +7% |
| Gardasil Revenue | $1.0B | $1.6B | -35% |
| Winrevair Revenue | $467M | $149M | +213% |
| Capvaxive Revenue | $279M | $47M | N/M |
New Launches Gaining Traction
Beyond Keytruda, Merck's newer products showed strong momentum:
Winrevair (pulmonary arterial hypertension): $1.4 billion in full-year sales, up from $419 million in the prior year, with Q4 sales of $467 million . The drug has now treated over 9,100 patients with 110,000+ prescriptions dispensed .
Capvaxive (pneumococcal vaccine): $759 million in 2025, ramping quickly from the 2024 launch .
Welireg (renal cell carcinoma): Sales increased 37% to $220 million in Q4 .
Animal Health: The segment delivered $6.4 billion in 2025 revenue, up 8%, with strong livestock performance .
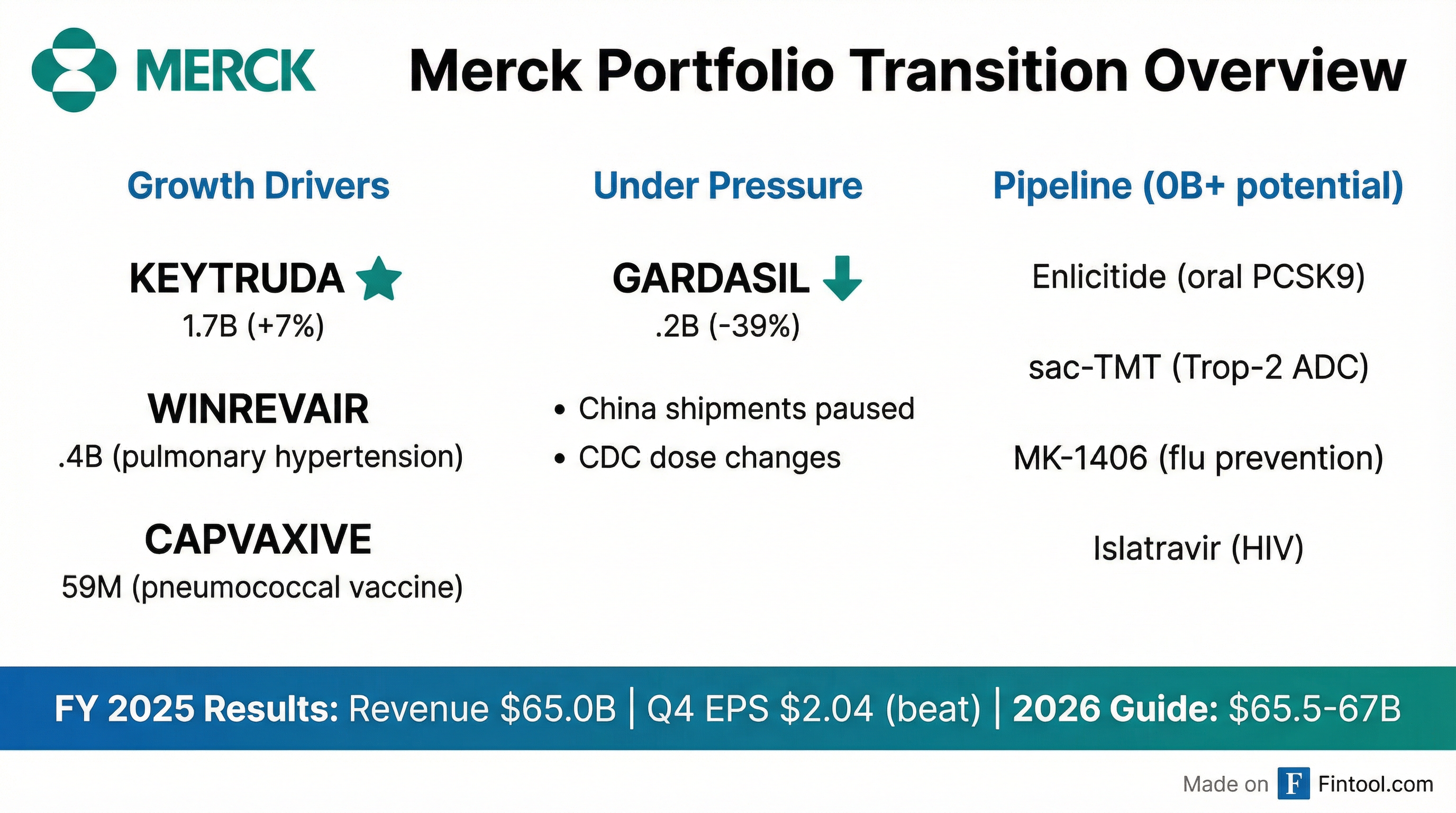
The $70 Billion Pipeline Promise
Management attempted to shift investor focus toward long-term growth, highlighting $70 billion in "potential commercial opportunity" by the mid-2030s—$20 billion more than a year ago and more than double the projected Keytruda peak of $35 billion .
CEO Rob Davis emphasized: "10 of these programs could be substantially clinically de-risked over the next two years and represent the majority of our $70 billion of non-risk adjusted commercial opportunity by the mid-2030s" .
Key pipeline catalysts in 2026 include:
- Islatravir + Lenacapavir: Potentially first once-weekly oral HIV treatment (PDUFA April 28)
- Enlicitide: Oral PCSK9 inhibitor with ACC presentation in March; outcomes study ongoing
- Tulisokibart: TL1A inhibitor with phase 3 ulcerative colitis data expected
- MK-3000: Novel WNT agonist for diabetic macular edema (phase 3 data)
- MK-1406: First-in-class flu prevention antiviral (phase 3 completion)
"We're entering a particularly robust period of first-time phase 3 data readouts from novel candidates," said Dean Li, President of Research Labs .
The recent $2.8 billion acquisition of Cidara Therapeutics added MK-1406, a strain-agnostic flu prevention antiviral that Merck believes has "greater than $5 billion in revenue potential" .
2026 Guidance: The Headwinds
The 2026 outlook reflects multiple pressures beyond Gardasil:
| Item | 2026 Impact |
|---|---|
| Generic competition (Januvia, Bridion, Dificid) | $2.5B headwind |
| IRA price setting | Included in headwind |
| Koselugo restructured agreement | Included in headwind |
| Lagevrio (COVID antiviral) | "Significantly lower sales" |
| Cidara acquisition charge | $3.65/share one-time |
Excluding the Cidara charge and ongoing deal costs, Merck's adjusted 2026 EPS midpoint would be $9.03 rather than $5.08 .
The company also announced approximately $3 billion in planned share repurchases for 2026 .
What to Watch
Near-term catalysts:
- February 20: FDA decision on Keytruda for platinum-resistant ovarian cancer
- March: ACC presentations on Winrevair (Cadence study) and Enlicitide
- April 28: PDUFA date for Islatravir + Doravirine HIV treatment
Longer-term questions:
- Can Merck convert its $70 billion pipeline into actual revenue before the Keytruda cliff?
- Will Gardasil ever return to meaningful China revenue, or is local competition permanent?
- How will the IRA's price negotiations impact the portfolio starting in 2029?
For now, Merck is a tale of two franchises: a cancer drug that continues to set records, and a vaccine that went from crown jewel to cautionary tale in under two years. The stock's 4% rally today suggests investors believe the pipeline can fill the gap—but that thesis won't be fully tested for another 2-3 years.