Merck Emerges as Lead Suitor for Revolution Medicines at $28-32 Billion
January 9, 2026 · by Fintool Agent

Merck has emerged as the lead suitor to acquire cancer drugmaker Revolution Medicines in a deal valued between $28 billion and $32 billion, according to the Financial Times, a dramatic escalation from earlier reports that Abbvie was pursuing the company at a lower valuation.
If consummated, this would be the largest pharmaceutical acquisition since Pfizer's $43 billion purchase of Seagen in 2023—and a defining move in Merck's strategy to diversify ahead of Keytruda's 2028 patent expiration.
Revolution shares jumped 16% in after-hours trading on the news, extending a stunning run that has seen the stock more than triple from its September 2025 lows.
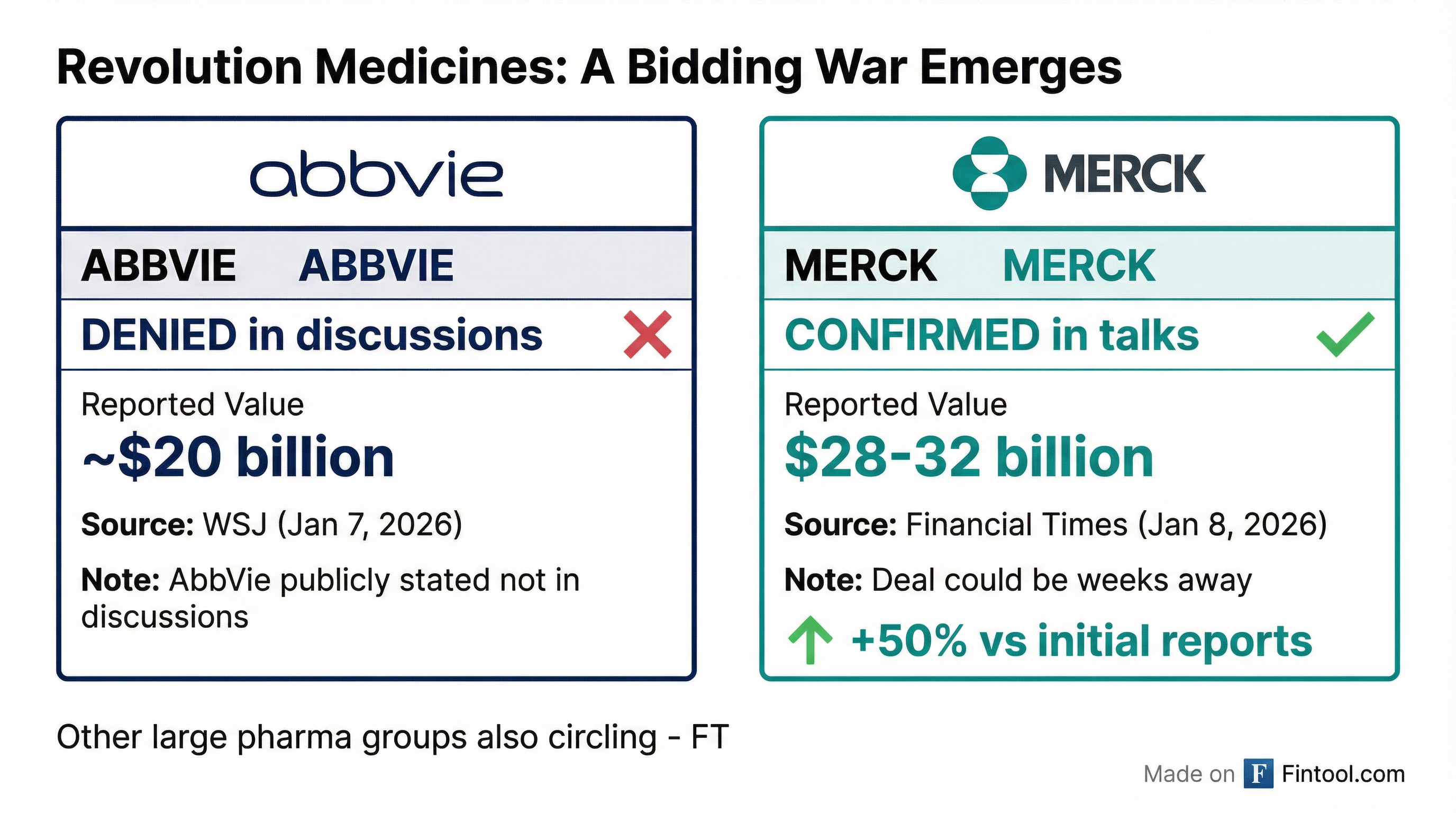
The Bidding War: From AbbVie to Merck
The situation evolved rapidly over 48 hours. On January 7, the Wall Street Journal reported that AbbVie was in advanced talks to acquire Revolution Medicines for approximately $20 billion. But AbbVie swiftly denied the report, telling Reuters it "is not in discussions with Revolution Medicines."
Now Merck has emerged with a significantly higher offer—representing a 40-60% premium to the earlier AbbVie reports. The Financial Times noted that the deal is not yet finalized and could be "at least several weeks away." Other large pharmaceutical companies are still circling, and another suitor could yet prevail.
Why Merck Needs This Deal
The strategic rationale for Merck is clear: Keytruda's patent cliff is approaching, and the company needs to diversify its oncology portfolio urgently.
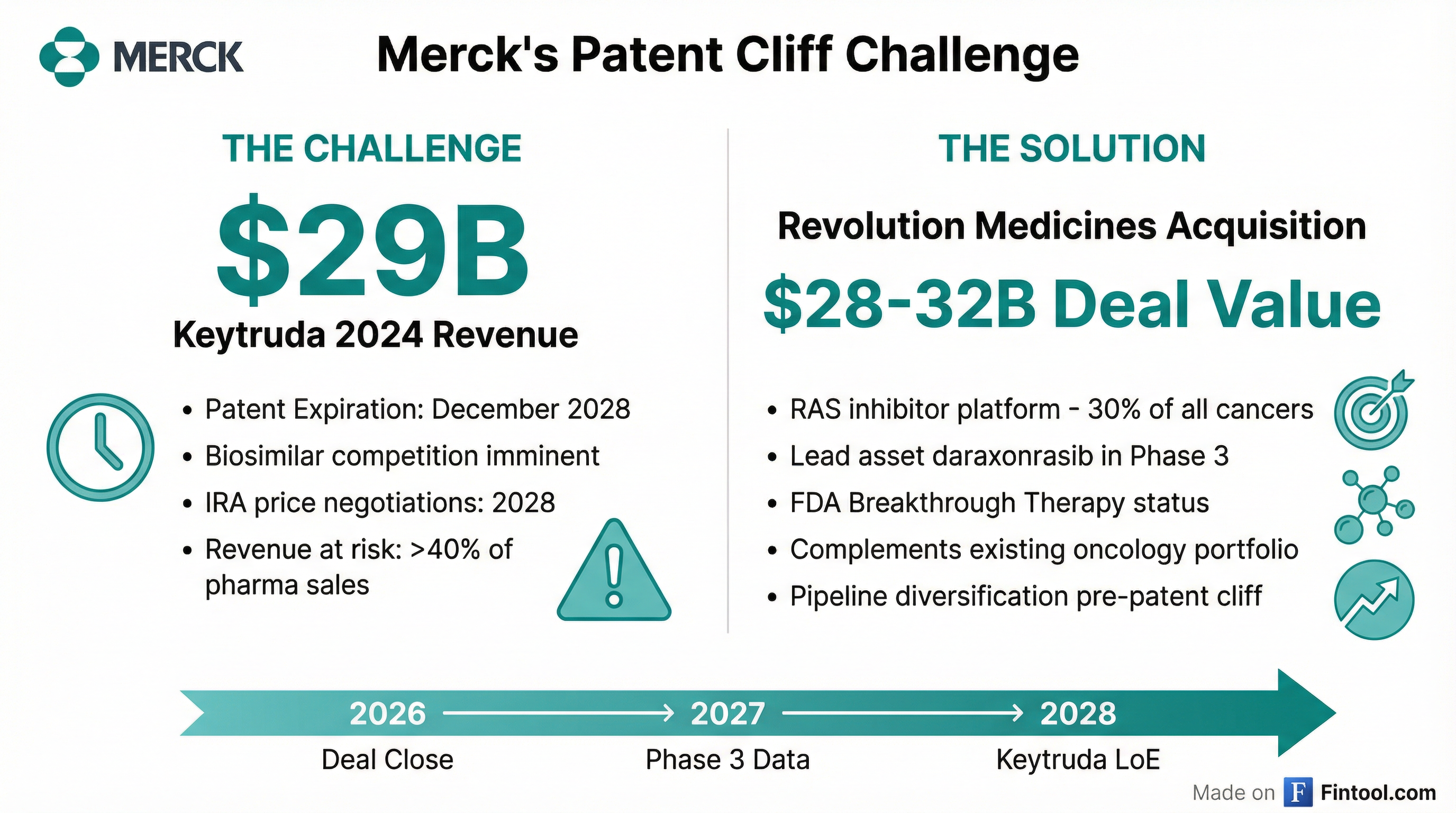
Keytruda generated $8.1 billion in Q3 2025 alone, up 8% year-over-year, driven by strong demand across metastatic indications and robust uptake in earlier-stage cancers. For full-year 2024, Keytruda generated approximately $29 billion in revenue—accounting for roughly 40% of Merck's pharmaceutical sales.
But that dominance creates existential risk:
| Threat | Timeline | Impact |
|---|---|---|
| U.S. Patent Expiration | December 2028 | Opens door to biosimilar competition |
| IRA Price Negotiations | Selection 2026, Effective 2028 | Government-set pricing kicks in |
| European Exclusivity | January 2031 | Delayed but inevitable |
| Biosimilar Development | Multiple programs in Phase 3 | Celltrion, Samsung Bioepis, Amgen in late stages |
S&P Global Ratings has flagged Merck's product concentration as a "material weakness," with more than half of 2024 pharma revenue coming from a single product. The company is set to lose nearly $45 billion in revenue-generating products by 2030.
On the Q3 2025 earnings call, CFO Caroline Litchfield emphasized the company's BD strategy: "Business development remains a high priority, and we are well-positioned to pursue additional science-driven value-enhancing transactions."
Revolution's Prize: The RAS Oncology Platform
Revolution Medicines has built what may be the industry's most advanced platform for targeting RAS mutations—genetic alterations present in approximately 30% of all human cancers and historically considered "undruggable."
The company's lead asset, daraxonrasib (RMC-6236), is a multi-selective RAS(ON) inhibitor with FDA Breakthrough Therapy and Orphan Drug designations for pancreatic cancer.
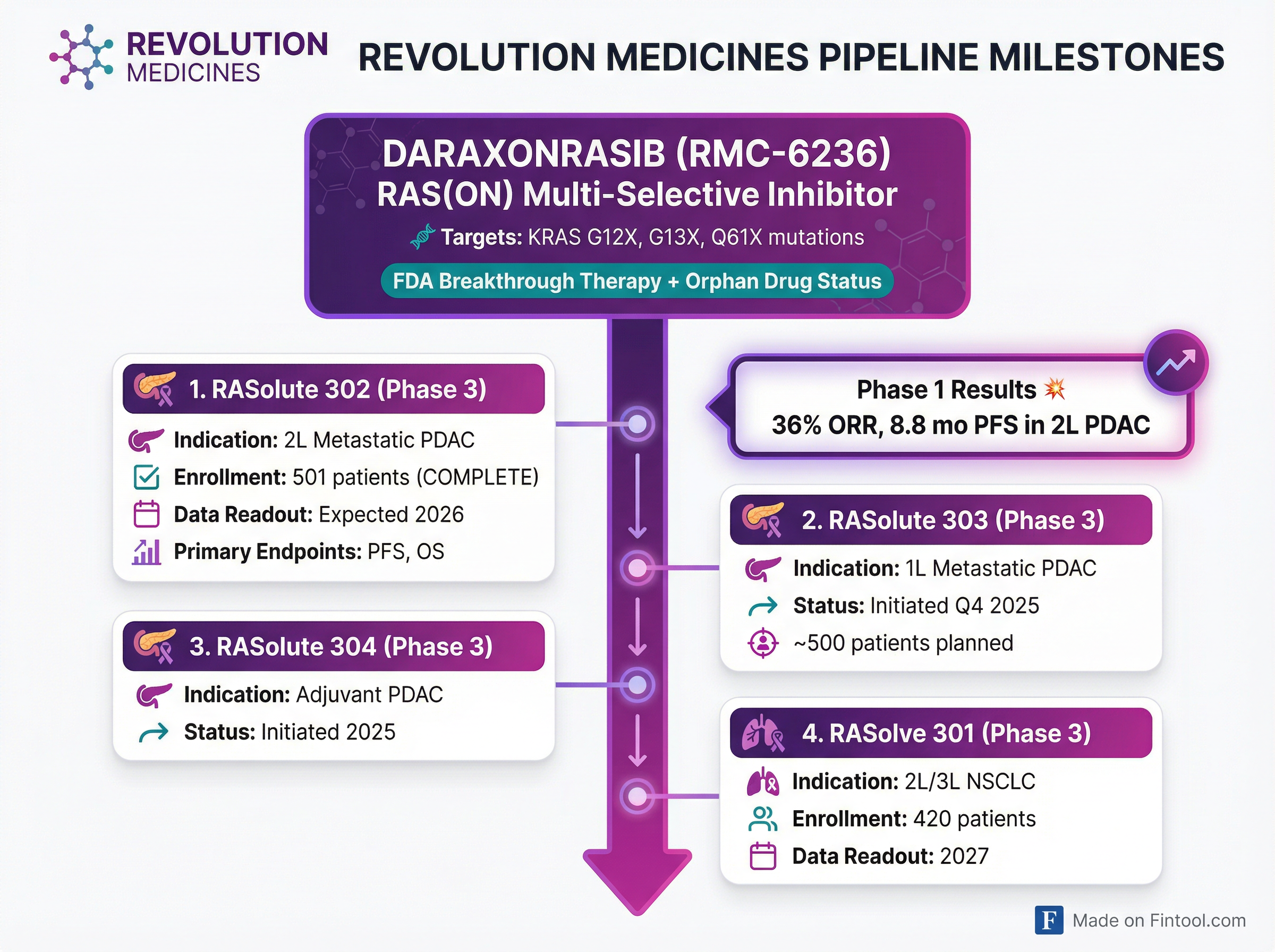
Phase 3 Clinical Program
| Trial | Indication | Status | Data Timeline |
|---|---|---|---|
| RASolute 302 | 2L Metastatic PDAC | 501 patients enrolled (complete) | Expected 2026 |
| RASolute 303 | 1L Metastatic PDAC | Initiated Q4 2025 | TBD |
| RASolute 304 | Adjuvant PDAC | Initiated 2025 | TBD |
| RASolve 301 | 2L/3L NSCLC | 420 patients enrolling | Expected 2027 |
Clinical Data Driving the Premium
Phase 1/1b results have been compelling. In second-line PDAC patients with KRAS G12X mutations treated at the 300mg dose:
- Objective response rate: 36%
- Median progression-free survival: 8.8 months
- Disease control rate: 91%
For context, current standard-of-care chemotherapy in second-line PDAC delivers median PFS of approximately 3-4 months with ORRs in the mid-single digits.
Mizuho analysts estimate Revolution's portfolio of RAS inhibitors could generate more than $10 billion in potential risk-adjusted global sales by 2035.
Stock Reaction: A 240% Rally Extends
Revolution shares have delivered extraordinary returns as M&A speculation intensified:
| Date | Event | Price | Change |
|---|---|---|---|
| September 2025 | 52-week low | $30 | - |
| January 7, 2026 | AbbVie report | $102.71 | +27% |
| January 8, 2026 | AbbVie denies, Merck emerges | $107.39 | +13% |
| January 9, 2026 | Continued speculation | $114.51 | +7% |
Current market cap: ~$22 billion, with a 52-week range of $29.17 to $120.49. The stock is now trading at 155% above its 50-day moving average of $73.85.
Merck's Recent Dealmaking
Merck has been actively diversifying through acquisitions:
| Deal | Date | Value | Strategic Rationale |
|---|---|---|---|
| Verona Pharma | 2025 | $10B | COPD drug ensifentrine |
| Cidara Therapeutics | 2025 | $9.2B | Influenza prevention |
| Acceleron | 2021 | $11.5B | PAH drug Winrevair |
| Harpoon Therapeutics | 2024 | $680M | T-cell engagers |
"The success of Keytruda has enabled us to build a diversified oncology pipeline," said Dean Li, President of Research Laboratories, on the Q3 2025 call.
What to Watch
- Deal terms and timing: FT suggests weeks away—pricing, structure, and regulatory considerations remain
- Competing bids: Other large pharma companies are reportedly still evaluating Revolution
- RASolute 302 data: Phase 3 readout expected in 2026 could influence final valuation
- Regulatory path: Revolution's breakthrough designations may support expedited review timelines
- Integration strategy: How Merck plans to advance the broader RAS(ON) portfolio
Related: