Royalty Pharma Bets $500M on Teva's Vitiligo Antibody, Targeting Market With Only One Approved Drug
January 11, 2026 · by Fintool Agent

Royalty Pharma is committing up to $500 million to accelerate development of Teva's anti-IL-15 antibody TEV-408 for vitiligo—a skin condition affecting over 8 million patients in major markets with only one FDA-approved treatment option.
The deal announced Sunday marks the second collaboration between the companies and represents a strategic bet on a differentiated mechanism in a market dominated by JAK inhibitors. If approved, TEV-408 would be the first systemic therapy for vitiligo.
Deal Structure
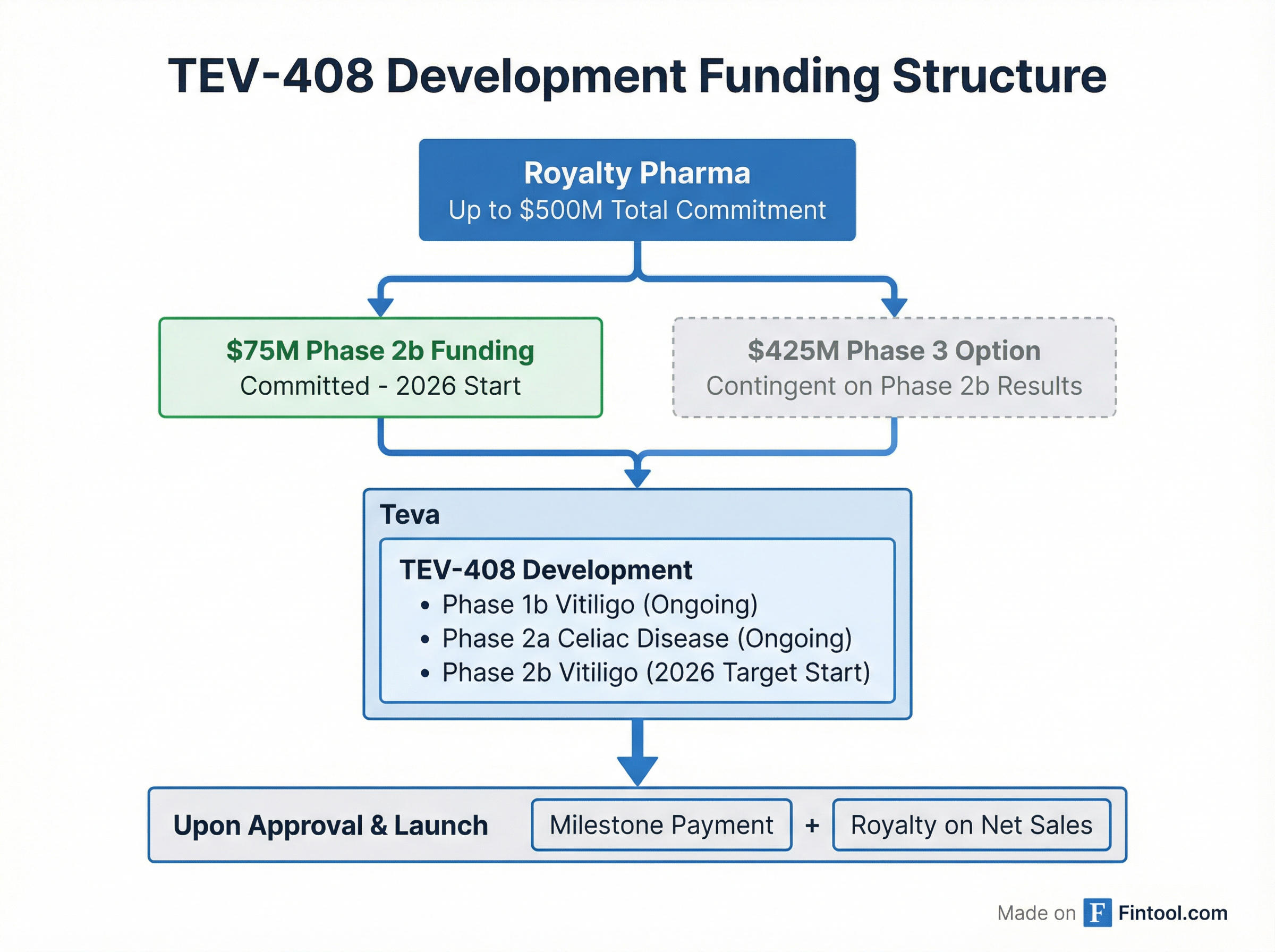
Royalty Pharma will fund development in two tranches:
| Component | Amount | Status |
|---|---|---|
| Phase 2b funding | $75 million | Committed—trial targeted to start 2026 |
| Phase 3 option | $425 million | Contingent on Phase 2b results |
| Total potential | $500 million |
Upon approval and launch, Teva will pay Royalty Pharma a milestone payment and royalties on worldwide net sales. Trial results are expected during 2026.
Why IL-15 Matters
TEV-408 takes a fundamentally different approach than existing therapies. While Incyte's Opzelura targets JAK1/JAK2, Teva's antibody directly blocks interleukin-15—a cytokine that drives the immune system's attack on melanocytes, the cells that produce skin pigment.
The therapy is currently in:
- Phase 1b for vitiligo (NCT06625177)
- Phase 2a for celiac disease (NCT06807463)
- Fast Track designation from the FDA (granted May 2025)
TEV-408 features a prolonged half-life with planned self-administration, potentially offering patient convenience advantages over daily topical applications or more frequent dosing.
Vitiligo: A Underserved $1-2 Billion Market
Vitiligo affects approximately 1% of the global population, yet treatment options remain severely limited. The condition causes significant psychosocial burden with only one FDA-approved pharmacologic treatment for repigmentation.
| Metric | Value |
|---|---|
| Global prevalence | 1 in 50 to 1 in 250 people |
| 7MM market size (2025) | $400-440 million |
| Projected market size (2034) | $900 million - $2 billion |
| CAGR | 3.7% - 9.6% |
| Diagnosis rate | 40-60% |
| Treatment rate | 10% |
*Market size estimates vary by source: *
The low treatment rate reflects the historical lack of effective options—most patients manage symptoms with phototherapy or cosmetic solutions rather than disease-modifying therapies.
Competitive Landscape
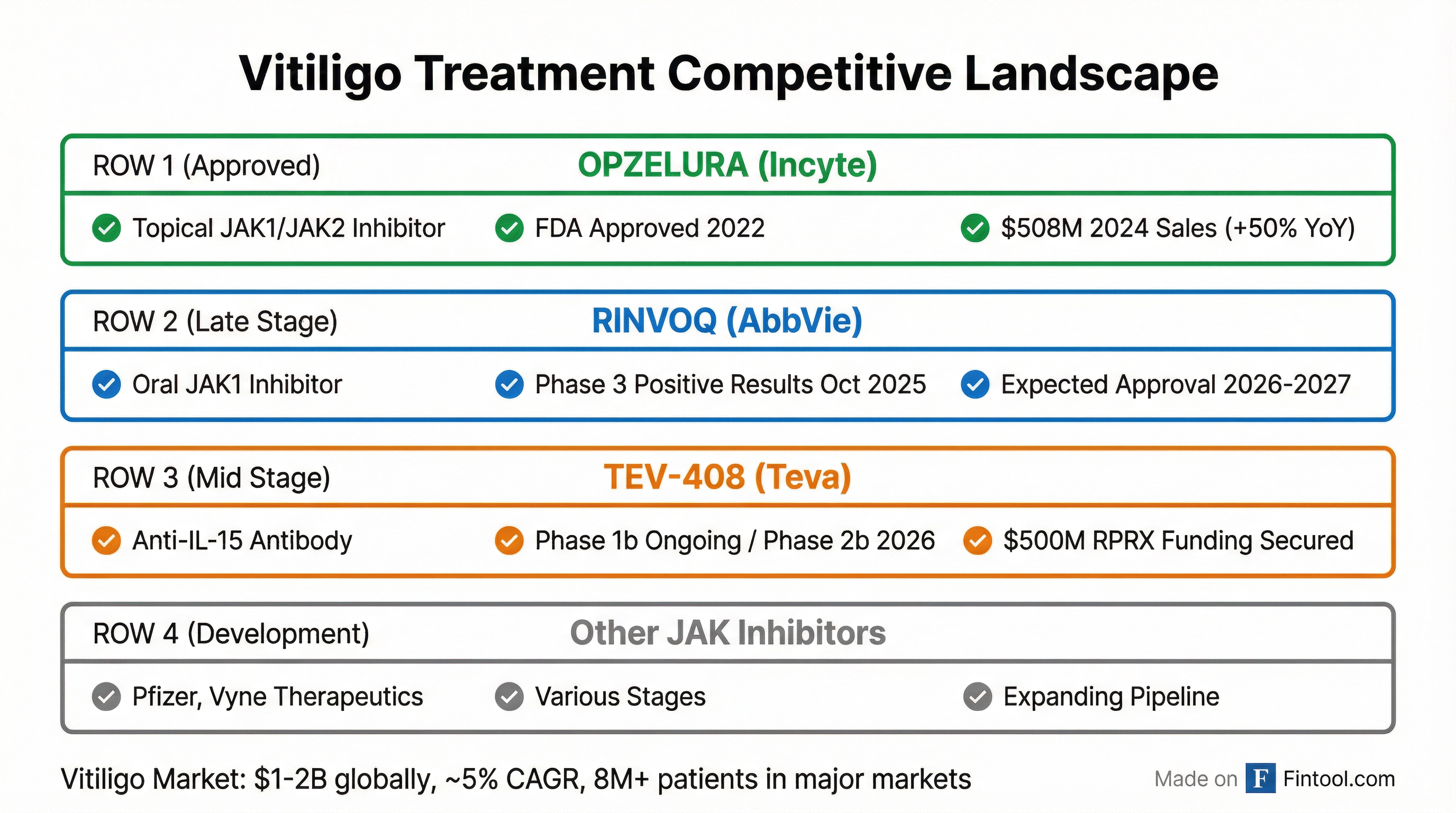
Incyte's Opzelura (ruxolitinib cream) currently owns the vitiligo treatment market:
| Year | Opzelura Sales | YoY Growth |
|---|---|---|
| 2023 | $338 million | - |
| 2024 | $508 million | +50% |
| 2025E | $630-670 million | +24-32% |
*Source: *
However, competition is intensifying:
-
Abbvie's RINVOQ (upadacitinib): Reported positive Phase 3 results in October 2025, with potential approval in 2026-2027. Unlike Opzelura's topical formulation, RINVOQ is an oral systemic therapy—a key differentiator.
-
Pfizer's LITFULO (ritlecitinib): In development for vitiligo after approval for alopecia areata.
TEV-408's anti-IL-15 mechanism represents a distinct approach from the JAK inhibitor class, potentially offering differentiated efficacy and safety profiles.
Strategic Fit for Both Companies
Teva's Pivot to Growth
The deal advances Teva's "Pivot to Growth" strategy, announced in May 2023, which emphasizes shifting from generics dependency toward innovative medicines.
Teva's recent financials show improving trajectory:
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue | $4.23B | $3.89B | $4.18B | $4.48B |
| Net Income | $(217)M | $214M | $282M | $433M |
| EBITDA Margin | 27.5% | 24.5% | 27.4% | 29.4% |
Values retrieved from S&P Global
Teva is also divesting its API business to sharpen focus on innovative and biosimilar medicines. CEO Richard Francis emphasized that "strategic collaborations fuel innovation" and highlighted vitiligo as a significant unmet need with "no systemic options" currently approved.
Royalty Pharma's Expansion
For Royalty Pharma, the deal extends its autoimmune disease exposure and continues a pattern of funding late-stage development in exchange for future royalties.
The company's Q3 2025 Portfolio Receipts reached $814 million, and it maintains a diversified royalty stream from products including Imbruvica, Tysabri, and Vertex's cystic fibrosis franchise.
CEO Pablo Legorreta called this "our second collaboration with Teva," underscoring the companies' growing relationship.
What to Watch
2026 Catalysts:
- Phase 2b trial initiation for vitiligo (targeted 2026)
- TEV-408 trial results disclosure
- AbbVie's potential RINVOQ approval for vitiligo
- Opzelura pediatric atopic dermatitis approval (expected H2 2025)
Key Questions:
- Can TEV-408's anti-IL-15 mechanism deliver superior or differentiated efficacy versus JAK inhibitors?
- Will systemic administration prove advantageous over topical or create safety hurdles?
- How will pricing and reimbursement compare in an increasingly competitive market?
The vitiligo landscape is rapidly evolving from a market with no approved treatments to one with multiple therapeutic classes competing for patients. Teva's $500 million bet—and Royalty Pharma's willingness to back it—suggests both see opportunity in a differentiated mechanism.