Vanda Pharma's NEREUS Becomes First New Motion Sickness Drug in 40 Years — Shares Surge 18%
December 31, 2025 · by Fintool Agent

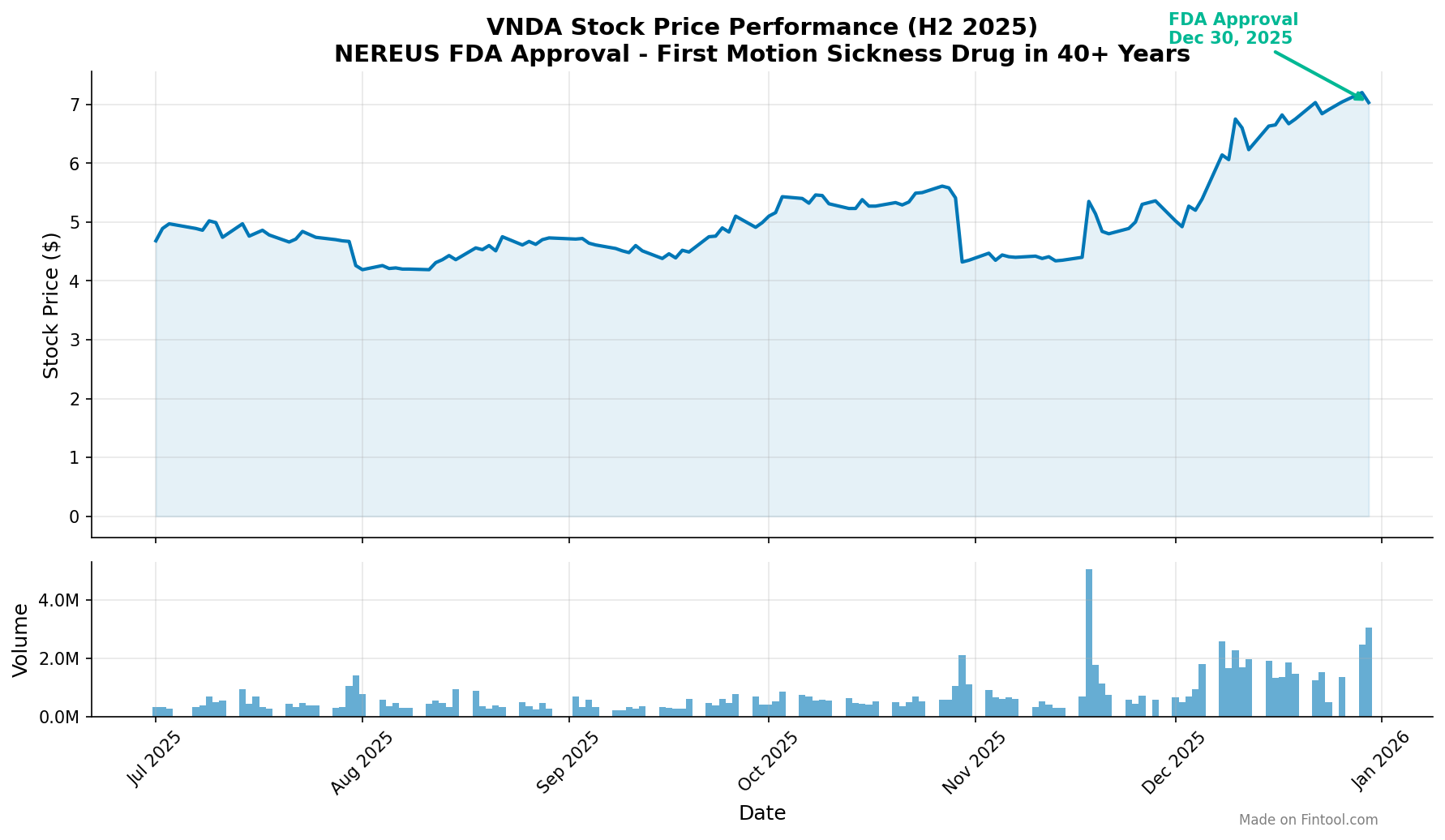
Vanda Pharmaceuticals secured FDA approval for NEREUS (tradipitant) on December 30, 2025 — the first new pharmacologic treatment for motion sickness in over four decades . Shares jumped as much as 18% in premarket trading on the news, with the stock touching three-year highs near $8.29 before settling at $7.03 by market close .
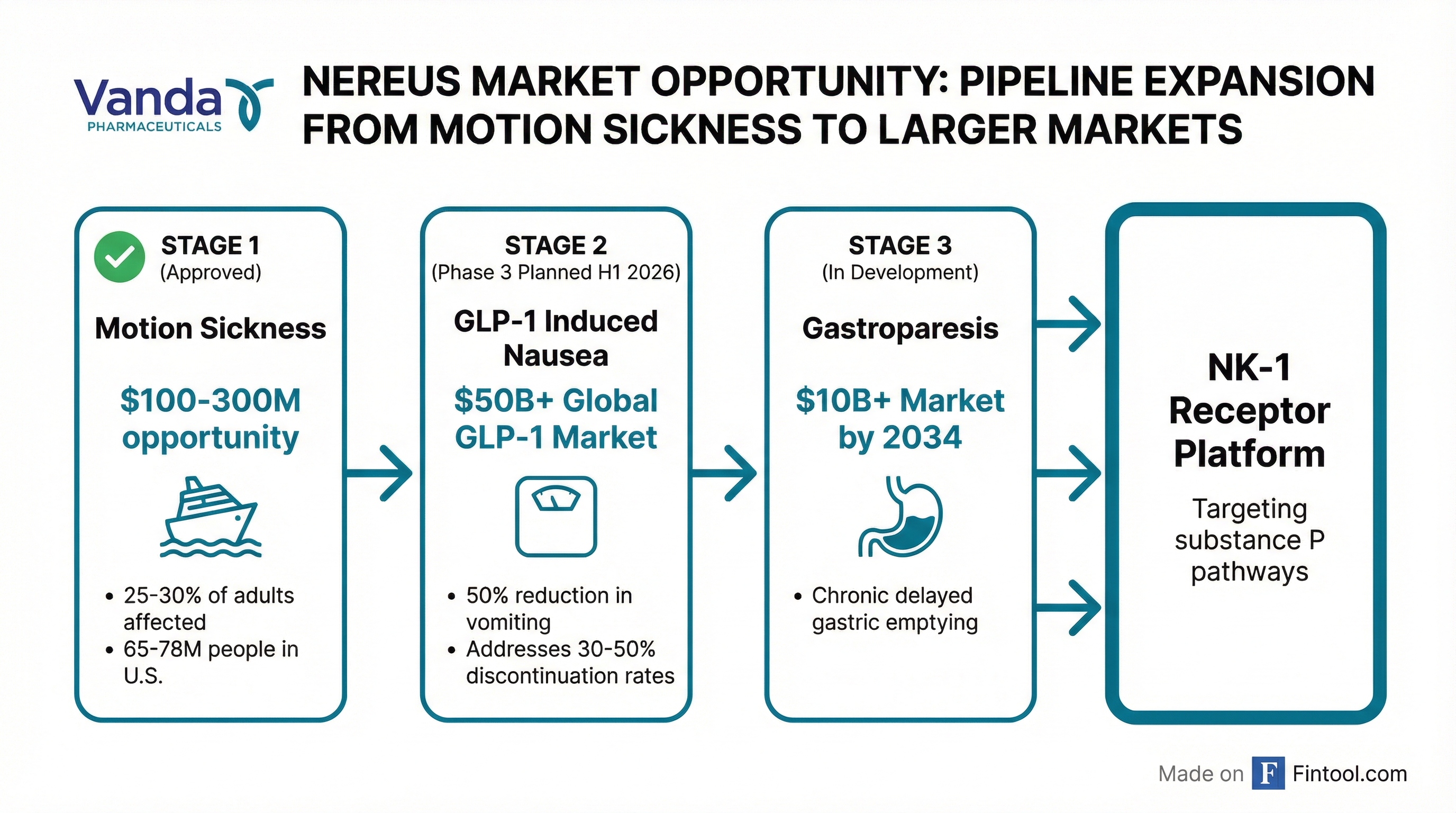
The approval marks a historic scientific milestone: no new drug for motion sickness had reached the market since the 1980s, leaving patients reliant on scopolamine patches and over-the-counter options like Dramamine . More significantly for investors, the approval validates Vanda's NK-1 receptor platform and positions tradipitant as a potential solution for GLP-1-induced nausea — a far larger commercial opportunity tied to the $50 billion obesity drug boom .
Clinical Data: 50-70% Reduction in Vomiting
NEREUS demonstrated robust efficacy across two pivotal Phase 3 trials conducted on boats with patients who had documented histories of motion sickness :
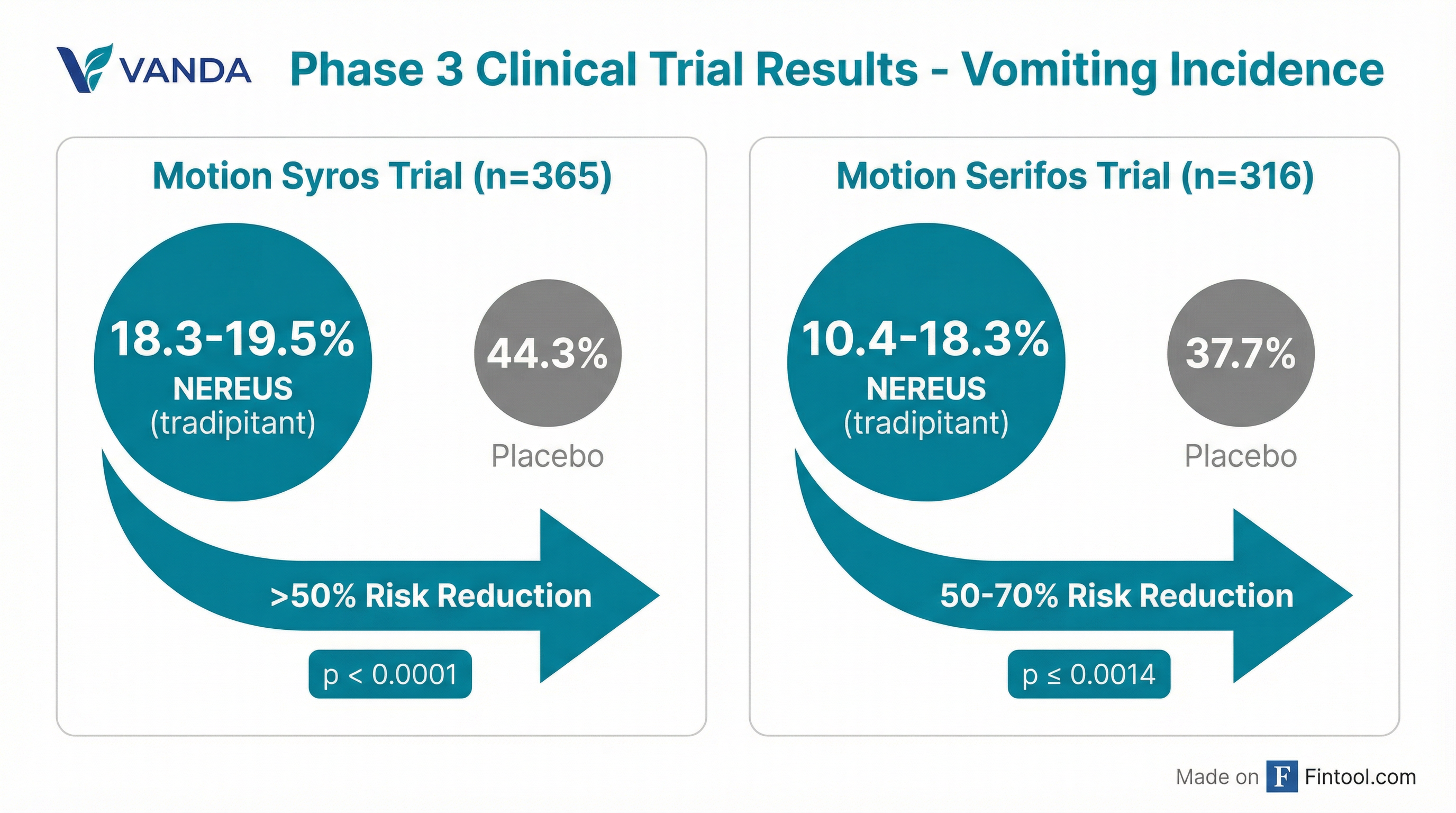
| Trial | Patients | NEREUS Vomiting Rate | Placebo Vomiting Rate | P-Value | Risk Reduction |
|---|---|---|---|---|---|
| Motion Syros | 365 | 18.3-19.5% | 44.3% | <0.0001 | >50% |
| Motion Serifos | 316 | 10.4-18.3% | 37.7% | ≤0.0014 | 50-70% |
The drug works by selectively blocking NK-1 receptors in the central nervous system, targeting the substance P pathway that triggers nausea and vomiting during sensory conflicts . This mechanism differs fundamentally from existing treatments: scopolamine patches work through anticholinergic effects and come with side effects like dry mouth and drowsiness, while Dramamine is an antihistamine with sedative properties.
A Massive Addressable Market
Motion sickness affects approximately 25–30% of adults — roughly 65–78 million people in the United States alone . While most cases are mild, an estimated 5–15% of the population experiences severe, recurrent symptoms that significantly impact quality of life .
"Sales of tradipitant solely in this indication could exceed $100 million annually at peak in the U.S. alone," said H.C. Wainwright analyst Raghuram Selvaraju .
Jefferies is more bullish, raising its price target to $7.50 from $5.00 and projecting NEREUS could be a $100-300 million opportunity . The firm notes Vanda plans a patient-centric digital marketing strategy targeting the 2-3 million U.S. patients currently taking Dramamine monthly .
The Bigger Opportunity: GLP-1-Induced Nausea
While motion sickness provides a solid commercial foundation, the real excitement lies in tradipitant's potential as an adjunct therapy for GLP-1 drugs like Novo Nordisk's Wegovy and Eli Lilly's Zepbound.
In a Phase 2 study announced in November 2025, tradipitant cut vomiting by 50% versus placebo in overweight and obese adults receiving Wegovy . The results are particularly compelling given that 30-50% of GLP-1 patients discontinue treatment — often due to gastrointestinal side effects before reaching therapeutic doses .
| Endpoint | Placebo (N=58) | Tradipitant (N=58) | P-Value |
|---|---|---|---|
| Proportion with vomiting | 58.6% | 29.3% | 0.0016 |
| Proportion with vomiting + moderate nausea | 48.3% | 22.4% | 0.0039 |
The global GLP-1 agonist market exceeded $50 billion through the first nine months of 2025 . A Phase 3 program for this indication is planned for the first half of 2026 .
Stock Performance and Valuation
VNDA shares have rallied significantly in anticipation of the approval, with the stock up approximately 45% year-to-date .
| Metric | Q4 2024 | Q1 2025 | Q2 2025 | Q3 2025 |
|---|---|---|---|---|
| Revenue ($M) | $53.2 | $50.0 | $52.6 | $56.3 |
| Net Income ($M) | -$4.9 | -$29.5 | -$27.2 | -$22.6 |
| Cash ($M) | $102.3 | $111.8 | $81.0 | $70.0 |
With a market cap of approximately $425 million and minimal debt ($10.9M)*, Vanda trades at a fraction of what the GLP-1 nausea opportunity alone could be worth. The company's existing hypercortisolism business generated $56.3 million in Q3 2025 revenue , providing a foundation while the NEREUS franchise scales.
Regulatory History: A Long Road
The approval caps a protracted regulatory journey. The FDA originally placed a partial clinical hold on tradipitant in December 2018, requiring additional six-month chronic toxicity studies in dogs because regulators initially classified motion sickness as a chronic condition .
The hold was lifted on December 4, 2025 after the FDA reclassified motion sickness as an acute condition, eliminating the study requirement . This cleared the path for the December 30 approval, meeting the PDUFA target action date .
Vanda originally licensed tradipitant from Eli Lilly in 2012 .
Competition and Launch Strategy
NEREUS enters a market with established, inexpensive alternatives:
- Transderm Scop (Viatris) — Prescription scopolamine patch
- Bonine (WellSpring Pharmaceutical) — OTC meclizine
- Dramamine (Prestige Consumer Healthcare) — OTC dimenhydrinate
Jefferies notes that launch adoption will be sensitive to out-of-pocket costs, as Dramamine is inexpensive . However, NEREUS offers a modern mechanism without the sedation issues that plague existing treatments.
Vanda plans to launch ahead of the 2026 summer travel season and will pursue a patient-centric digital marketing strategy rather than building a large prescriber-focused sales force .
What to Watch
-
NEREUS Launch Execution — Pricing, insurance coverage, and early prescription data will be critical signals for the motion sickness franchise in H1 2026
-
GLP-1 Phase 3 Initiation — Vanda expects to begin Phase 3 trials for tradipitant as an adjunct to GLP-1 drugs in the first half of 2026
-
Gastroparesis Progress — FDA administrative proceedings regarding the gastroparesis indication resume January 7, 2026
-
Cash Runway — With $70 million in cash and ongoing losses, Vanda may need to raise capital to fund the GLP-1 Phase 3 program
*Values retrieved from S&P Global
Related: Vanda Pharmaceuticals · Eli Lilly · Novo Nordisk · Viatris · Prestige Consumer Healthcare