Earnings summaries and quarterly performance for ESTABLISHMENT LABS HOLDINGS.
Executive leadership at ESTABLISHMENT LABS HOLDINGS.
Board of directors at ESTABLISHMENT LABS HOLDINGS.
Research analysts who have asked questions during ESTABLISHMENT LABS HOLDINGS earnings calls.
Anthony Petrone
Mizuho Group
6 questions for ESTA
Joshua Jennings
TD Cowen
6 questions for ESTA
Allen Gong
JPMorgan Chase & Co.
4 questions for ESTA
Mason Carrico
Stephens Inc.
4 questions for ESTA
Sam Eiber
BTIG, LLC
4 questions for ESTA
Matthew Taylor
Jefferies
3 questions for ESTA
Mike Matson
Needham & Company, LLC
3 questions for ESTA
Joanne Wuensch
Citigroup Inc.
2 questions for ESTA
K. Gong
JPMorgan Chase & Co.
2 questions for ESTA
Marie Thibault
BTIG
2 questions for ESTA
Caitlin Cronin
Canaccord Genuity
1 question for ESTA
Caitlin Roberts
Canaccord Genuity
1 question for ESTA
Matt Taylor
Jefferies & Company Inc.
1 question for ESTA
Michael Matson
Needham & Company
1 question for ESTA
Recent press releases and 8-K filings for ESTA.
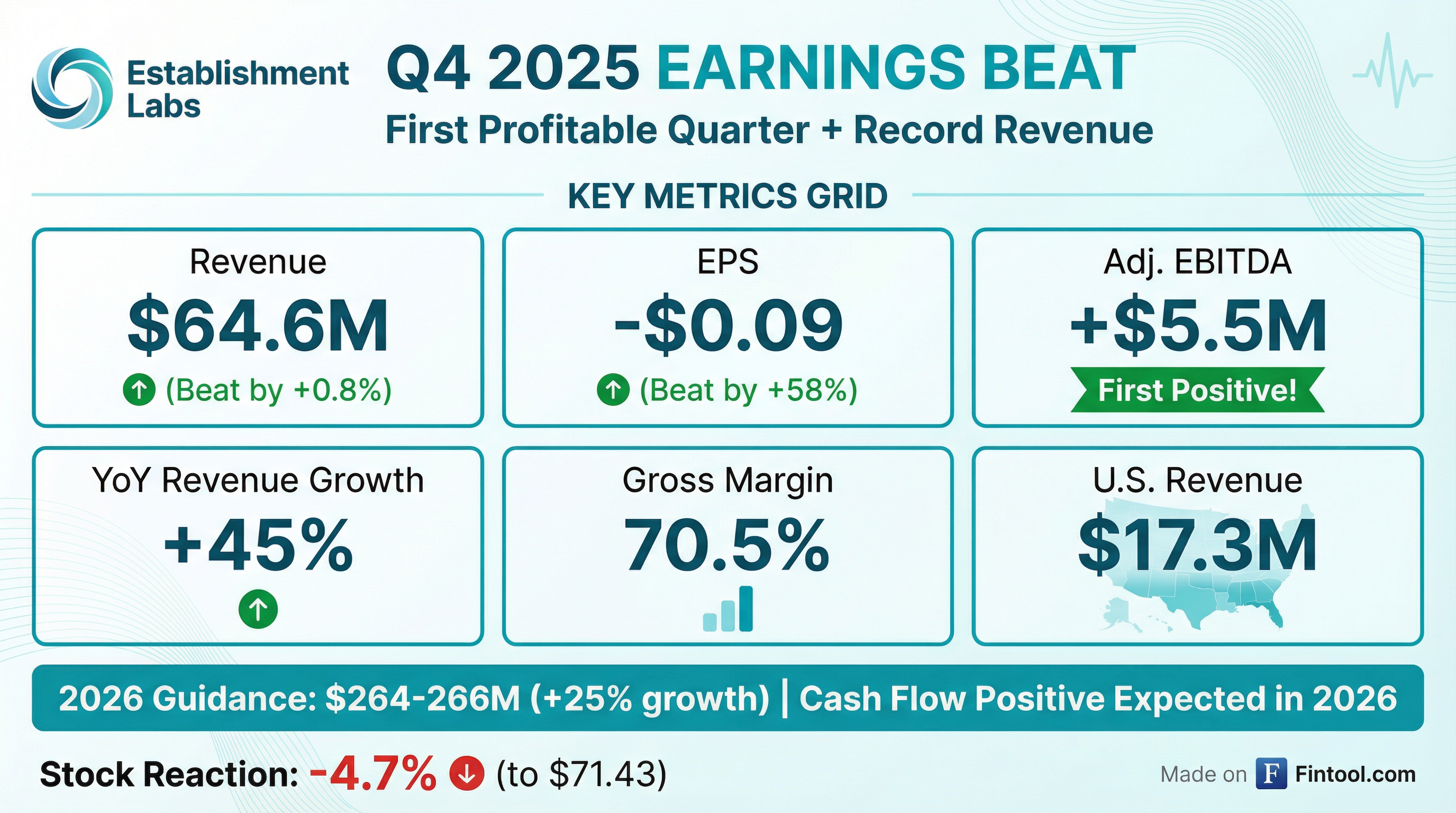
- Establishment Labs Holdings reported Q4 2025 revenue of $64.6 million, a 45.2% increase year-over-year, contributing to a full-year 2025 revenue of $211.1 million, up 27.2% from 2024.
- The company achieved positive Q4 2025 adjusted EBITDA of $5.5 million, a significant improvement from a -$13.1 million loss in Q4 2024, and ended 2025 with a cash balance of $75.6 million.
- For 2026, revenue guidance is set at $264 million-$266 million, indicating 25.1%-26% growth over 2025, with expectations for adjusted EBITDA to be positive every quarter and the company to become cash flow positive this year. Gross margins are also expected to increase by 200-300 basis points.
- The U.S. market was a key growth driver, with Motiva revenue reaching $45.6 million in 2025 and achieving an approximate 20% augmentation market share by the end of 2025. The company plans to expand its U.S. sales force with up to 15 new representatives in 2026. Additionally, Raj Denhoy is transitioning from CFO to a new role leading global strategy, with Sandra Harris taking over as CFO.
- Establishment Labs reported Q4 2025 revenue of $64.6 million, a 45.2% increase versus Q4 2024, contributing to full-year 2025 revenue of $211.1 million, up 27.2% over 2024.
- The company achieved positive adjusted EBITDA of $5.5 million in Q4 2025, a significant improvement from a $13.1 million loss in Q4 2024, marking its second consecutive quarter of positive adjusted EBITDA.
- For 2026, revenue guidance is set at $264 million-$266 million, representing a 25.1%-26% increase over 2025, with expectations to be adjusted EBITDA positive every quarter and cash flow positive for the year.
- Preservé will move from early experience to a full launch in March 2026, expected to expand the market and contribute to higher gross margins.
- Raj Denhoy is transitioning from CFO to a new role leading global strategy, and Sandra Harris will take over as CFO.
- Establishment Labs reported Q4 2025 revenue of $64.6 million, a 45.2% increase over Q4 2024, bringing full-year 2025 total revenue to $211.1 million, an increase of 27.2% over 2024.
- The company achieved positive adjusted EBITDA of $5.5 million in Q4 2025, a significant improvement from a -$13.1 million loss in Q4 2024, and expects to be adjusted EBITDA positive every quarter in 2026, with the first positive cash flow quarter this year. The ending cash balance for 2025 was $75.6 million.
- For 2026, Establishment Labs provided revenue guidance of $264 million-$266 million, representing 25.1%-26% growth over 2025, with US sales expected to exceed 30% of overall sales.
- US Motiva revenue reached $45.6 million in 2025, securing an approximate 20% augmentation market share exiting 2025, and the company plans to expand its US sales force with up to 15 more sales representatives in 2026. The minimally invasive platform's global demand is expected to exceed $30 million in 2026.
- Raj Denhoy, CFO, is transitioning to a new global strategy role, and Cassandra Harris will take over as CFO, effective March 9th.
- Establishment Labs reported fourth quarter 2025 revenue of $64.6 million, a 45.2% increase from the prior year, and full year 2025 revenue of $211.1 million, up 27.2% over 2024.
- The company achieved a positive Adjusted EBITDA of $5.5 million in Q4 2025, an $18.6 million increase compared to a loss of $13.1 million in the year-ago period, and reduced its net loss from operations by 79% to $3.9 million.
- As of December 31, 2025, Establishment Labs held a cash balance of $75.6 million, an increase of $4.9 million from Q3 2025.
- For 2026, the company provided revenue guidance of $264 million to $266 million, representing a 25.1% to 26.0% increase over 2025, and projects at least 25% revenue growth for 2027.
- Establishment Labs reported fourth quarter 2025 revenue of $64.6 million, an increase of 45.2% from the year-ago period, and full year 2025 revenue of $211.1 million, up 27.2% over 2024.
- The company achieved a net loss from operations of $3.9 million in Q4 2025, a 79% reduction compared to the prior year, and recorded positive adjusted EBITDA of $5.5 million for the quarter.
- As of December 31, 2025, the cash balance was $75.6 million, an increase of $4.9 million compared to Q3 2025.
- For 2026, Establishment Labs provided revenue guidance of $264 million to $266 million, representing a 25.1% to 26.0% increase over 2025, and projects at least 25% revenue growth for 2027. The company anticipates its first quarter of positive cash flow in 2026 and full year positive cash flow in 2027.
- Establishment Labs reported a successful 2025, with pre-announced global revenue growth of 27% to between $210.5 million and $211.5 million, and achieved EBITDA positive in Q3 2025.
- The U.S. market launch was strong, generating over $45 million in revenue in its first year (2025) and securing an estimated market share of approximately 20% by year-end.
- The company projects a minimum of 25% growth in 2026 and 2027, driven by the minimally invasive Preservae platform (expected to contribute over $30 million in 2026), expansion into smaller implant sizes, and the upcoming Recon indication, which could double the addressable market.
- Profitability is a key focus, with gross profit margins now above 70% and the company anticipating becoming free cash flow positive in 2026.
- Establishment Labs (ESTA) reported 27% global growth in 2025, with revenue between $210.5 million and $211.5 million, and achieved over $45 million in U.S. revenue in its first year post-FDA approval.
- The company became EBITDA positive in Q3 2025 and expects to maintain this into Q4 2025, with a cash balance of $75.5 million at the end of 2025.
- ESTA anticipates at least 25% growth over the next two years (2026 and 2027) and expects to be cash flow positive in 2026.
- Key growth drivers for 2026 include the formal U.S. launch of the minimally invasive Preservae platform, projected to generate over $30 million in revenue, and the expansion of the U.S. sales force by up to 15 representatives.
- The company also achieved a gross margin above 70% in 2025, driven by higher-margin opportunities in the U.S. and direct markets.
- Establishment Labs reported 27% global revenue growth for 2025, with total revenue between $210.5 million and $211.5 million. The U.S. market, launched in 2025, generated over $45 million in its first year, achieving approximately 20% market share by the end of 2025.
- The company achieved EBITDA positive in Q3 2025 and expects to maintain this in Q4 2025, with its cash balance improving to $75.5 million by the end of 2025. Gross margin surpassed 70% in 2025, with expectations for continued significant margin expansion in the coming years.
- Management anticipates at least 25% revenue growth over the next two years (2026 and 2027) and is on track to be cash flow positive in 2026.
- Key growth drivers include the minimally invasive Preservé platform, projected to contribute over $30 million in revenue in 2026 , and the upcoming recon indication approval, which is expected to double the addressable U.S. market with a real commercial impact in 2027.
- Establishment Labs Holdings Inc. expects preliminary unaudited revenue for Q4 2025 to be between $64.0 million and $65.0 million.
- For fiscal year 2025, total revenue is anticipated to range from $210.5 million to $211.5 million, representing approximately 27% growth over 2024.
- The company's year-end 2025 cash position is expected to be approximately $75.5 million, an increase of $4.8 million from the end of the third quarter of 2025.
- CEO Peter Caldini highlighted record quarterly revenue and expects the company to become free cash flow positive later in 2026.
- Establishment Labs' preliminary unaudited revenue for Q4 2025 is expected to be between $64.0 million and $65.0 million, including $17.0 million to $17.5 million from Motiva sales in the United States.
- Preliminary unaudited revenue for fiscal year 2025 is expected to be in the range of $210.5 million to $211.5 million, representing approximately 27% growth over 2024.
- The company's year-end 2025 cash position is expected to be approximately $75.5 million, an increase of $4.8 million from the end of the third quarter of 2025.
- Management anticipates turning free cash flow positive later in 2026 and expects operating leverage to become evident in 2026 and beyond.
Quarterly earnings call transcripts for ESTABLISHMENT LABS HOLDINGS.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more