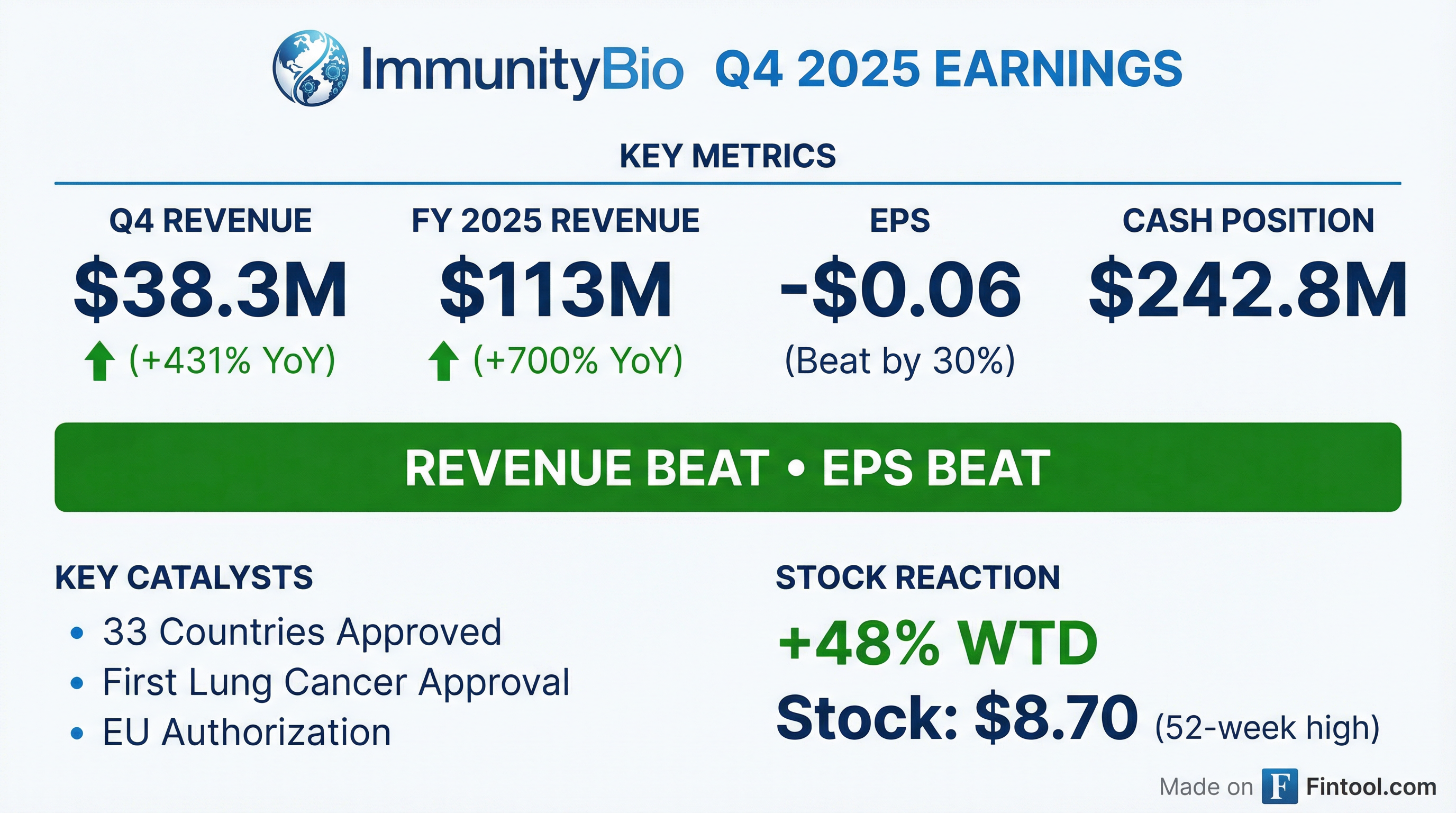
Earnings summaries and quarterly performance for ImmunityBio.
Executive leadership at ImmunityBio.
Board of directors at ImmunityBio.
Research analysts covering ImmunityBio.
Recent press releases and 8-K filings for IBRX.
ImmunityBio Completes Enrollment in Pivotal Trial for ANKTIVA® Plus BCG
IBRX
Product Launch
New Projects/Investments
- ImmunityBio (IBRX) has completed enrollment for its pivotal QUILT 2.005 trial, evaluating ANKTIVA® plus BCG versus BCG alone in 366 patients with BCG-naïve non-muscle invasive bladder cancer (NMIBC) carcinoma in situ.
- An interim analysis demonstrated a statistically significant improvement in the duration of complete response for ANKTIVA plus BCG, with 85% of patients maintaining a complete response at six months (compared to 57% for BCG alone) and 84% at nine months (compared to 52% for BCG alone), with no significant safety concerns observed.
- The company anticipates submitting a biologics license application (BLA) to the U.S. FDA by Q4 2026, with additional study results expected to be available in the same quarter.
2 days ago
ImmunityBio Partners for ANKTIVA® Launch in Saudi Arabia
IBRX
Product Launch
New Projects/Investments
- ImmunityBio has partnered with Biopharma and Cigalah Healthcare to launch its immunotherapy drug, ANKTIVA®, in Saudi Arabia and the broader Middle East and North Africa (MENA) region.
- The Saudi Food and Drug Authority (SFDA) approved ANKTIVA® in January 2026 for BCG-unresponsive non-muscle invasive bladder cancer and metastatic non-small cell lung cancer, with distribution anticipated within the next 60 days.
- To support its regional growth, ImmunityBio has established a wholly owned subsidiary in Saudi Arabia and is in discussions with regulatory authorities to expand ANKTIVA® indications.
8 days ago
ImmunityBio Expands ANKTIVA Access in Europe with New Partnership and Subsidiary
IBRX
Product Launch
New Projects/Investments
- ImmunityBio has partnered with Accord Healthcare to commercialize ANKTIVA® (nogapendekin alfa inbakicept) across the EU, UK, and European Free Trade Association members, with Accord deploying over 100 sales, medical, and marketing professionals.
- To support its European distribution and commercialization strategy, ImmunityBio has established an Irish subsidiary in Dublin.
- ANKTIVA® is now authorized across 33 countries, including the European Union (conditional marketing authorization granted in February 2026), for the treatment of BCG-unresponsive non-muscle invasive bladder cancer carcinoma in situ.
Feb 19, 2026, 2:00 PM
ImmunityBio Receives European Commission Authorization for ANKTIVA
IBRX
Product Launch
- ImmunityBio, Inc. received conditional marketing authorization from the European Commission for ANKTIVA® with BCG for the treatment of adult patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS), with or without papillary tumors.
- This authorization expands the global access of ANKTIVA to 33 countries, including all 27 EU member states plus Iceland, Norway, and Liechtenstein.
- ANKTIVA plus BCG is the first immunotherapy to receive marketing authorization in Europe for this NMIBC indication, addressing an unmet medical need for over 150,000 patients diagnosed annually across Europe.
- This approval follows previous authorizations in the United States (April 2024), United Kingdom (July 2025), and Kingdom of Saudi Arabia (January 2026), establishing a global commercial footprint across four regulatory jurisdictions in under two years.
Feb 18, 2026, 2:02 PM
ImmunityBio receives EU marketing authorization for ANKTIVA®
IBRX
Product Launch
New Projects/Investments
- ImmunityBio has received conditional marketing authorization from the European Commission for ANKTIVA® (nogapendekin alfa inbakicept) in combination with Bacillus Calmette-Guérin (BCG) for the treatment of adult patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS), with or without papillary tumors.
- This authorization makes ANKTIVA the first immunotherapy approved in Europe for this NMIBC indication, expanding its global reach to 33 countries across four regulatory jurisdictions.
- The approval addresses a significant unmet medical need for over 150,000 NMIBC patients diagnosed annually in Europe, who previously faced radical cystectomy as the primary alternative for BCG-unresponsive cases.
- Clinical data supporting the authorization from the QUILT-3.032 study showed a 71% complete response rate and a median duration of complete response of 26.6 months.
Feb 18, 2026, 2:00 PM
ImmunityBio Launches Phase 2 Clinical Study for Lymphoma Treatment
IBRX
Product Launch
New Projects/Investments
- ImmunityBio, Inc. (NASDAQ: IBRX) has initiated ResQ215B, a Phase 2 clinical study.
- The study evaluates a chemotherapy-free, lymphodepletion-free combination of its off-the-shelf CD19 CAR-NK cell therapy with ANKTIVA (nogapendekin alfa inbakicept) and rituximab.
- This treatment targets patients with indolent B-cell non-Hodgkin lymphoma (NHL), including Waldenström's Macroglobulinemia.
- The trial builds on Phase 1 QUILT-106 results, which demonstrated disease control in all four evaluable Waldenström's patients without lymphodepletion, including two complete remissions ongoing at 7 and 15 months.
- The treatment is designed for outpatient administration in 21-day cycles, eliminating the need for inpatient hospitalization.
Feb 17, 2026, 4:25 PM
ImmunityBio Reports Regulatory Engagement with Saudi Food and Drug Authority
IBRX
Product Launch
New Projects/Investments
- ImmunityBio engaged in regulatory discussions with the Saudi Food and Drug Authority (SFDA), which encouraged the company to submit a regulatory package for its recombinant BCG (rBCG) to address the global BCG shortage.
- The company initiated discussions with the SFDA for the expansion of ANKTIVA® in combination with checkpoint inhibitors for additional tumor types, following the SFDA's accelerated approval of ANKTIVA in January 2026 for metastatic non-small cell lung cancer (NSCLC) and for BCG unresponsive non-muscle invasive bladder cancer.
- To support its Middle East and North Africa strategy, ImmunityBio has established a wholly-owned subsidiary and plans to open a regional office in Saudi Arabia.
Feb 17, 2026, 11:07 AM
ImmunityBio Amends Convertible Promissory Note Terms
IBRX
Debt Issuance
- ImmunityBio, Inc. (IBRX) entered into a Convertible Note Amendment with Nant Capital, LLC on January 23, 2026.
- This amendment modifies a $505.0 million convertible promissory note, originally dated December 10, 2024.
- The key change allows Nant Capital, LLC, an entity affiliated with Dr. Patrick Soon-Shiong, to convert any portion of the outstanding principal amount of the note into common stock at any time prior to its maturity date.
Jan 26, 2026, 12:00 PM
ImmunityBio Reports Positive Phase 2 Clinical Results for Glioblastoma Treatment
IBRX
New Projects/Investments
- ImmunityBio announced updated Phase 2 clinical results for its chemotherapy-free ANKTIVA® Plus CAR-NK combination immunotherapy in recurrent glioblastoma, reporting that median overall survival has not yet been reached and 19 of 23 enrolled patients remain alive as of January 22, 2026.
- The treatment regimen demonstrated a manageable safety profile, with only three treatment-related serious adverse events reported among 41 glioblastoma patients across the QUILT-3.078 study and single-patient INDs.
- Patients, who presented with severe lymphopenia at enrollment (mean absolute lymphocyte count (ALC) of 0.9 x 10^3/uL), showed recovery and maintenance of lymphocyte counts, with mean ALC increasing to ≥ 1.4 x 10^3/uL within one treatment cycle.
Jan 23, 2026, 12:00 PM
ImmunityBio Advances Regulatory Discussions for ANKTIVA Resubmission
IBRX
Product Launch
New Projects/Investments
- ImmunityBio held discussions with the U.S. FDA regarding a potential resubmission path for its supplemental Biologics License Application (sBLA) for ANKTIVA® in BCG-unresponsive papillary bladder cancer.
- The FDA recommended the submission of additional information, not requiring new clinical trials, which ImmunityBio will provide within the next 30 days.
- Long-term data for ANKTIVA in papillary disease demonstrates approximately 96% bladder cancer-specific survival and greater than 80% bladder preservation at three years.
- ANKTIVA is currently approved in the U.S., U.K., E.U. (conditional), and Saudi Arabia for BCG-unresponsive NMIBC CIS with or without papillary tumors.
Jan 20, 2026, 9:00 AM
Fintool News
In-depth analysis and coverage of ImmunityBio.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more