Earnings summaries and quarterly performance for Summit Therapeutics.
Executive leadership at Summit Therapeutics.
Board of directors at Summit Therapeutics.
Research analysts who have asked questions during Summit Therapeutics earnings calls.
Mitchell Kapoor
H.C. Wainwright & Co.
8 questions for SMMT
Yigal Nochomovitz
Citigroup Inc.
8 questions for SMMT
Eric Schmidt
Cantor Fitzgerald & Co.
4 questions for SMMT
Mohit Bansal
Wells Fargo & Company
4 questions for SMMT
Reni Benjamin
Citizens JMP Securities
4 questions for SMMT
Asthika Goonewardene
Truist Securities
3 questions for SMMT
Brad Canino
Stifel Financial Corp.
3 questions for SMMT
David Dai
UBS Group AG
3 questions for SMMT
Kelly Shi
Jefferies
3 questions for SMMT
Tyler Van Buren
TD Cowen
3 questions for SMMT
Bradley Canino
Stifel
2 questions for SMMT
Cory Kasimov
Evercore ISI
2 questions for SMMT
Faisal Khursheed
Jefferies
2 questions for SMMT
Josh Schimmer
Cantor Fitzgerald
2 questions for SMMT
Karina Rabayeva
Truist Securities
2 questions for SMMT
Mark Schwartz
Goldman Sachs
2 questions for SMMT
Clara Dong
Jefferies
1 question for SMMT
Cory Kazimov
Evercore
1 question for SMMT
Dara Azar
Stifel
1 question for SMMT
Salveen Richter
Goldman Sachs
1 question for SMMT
Recent press releases and 8-K filings for SMMT.
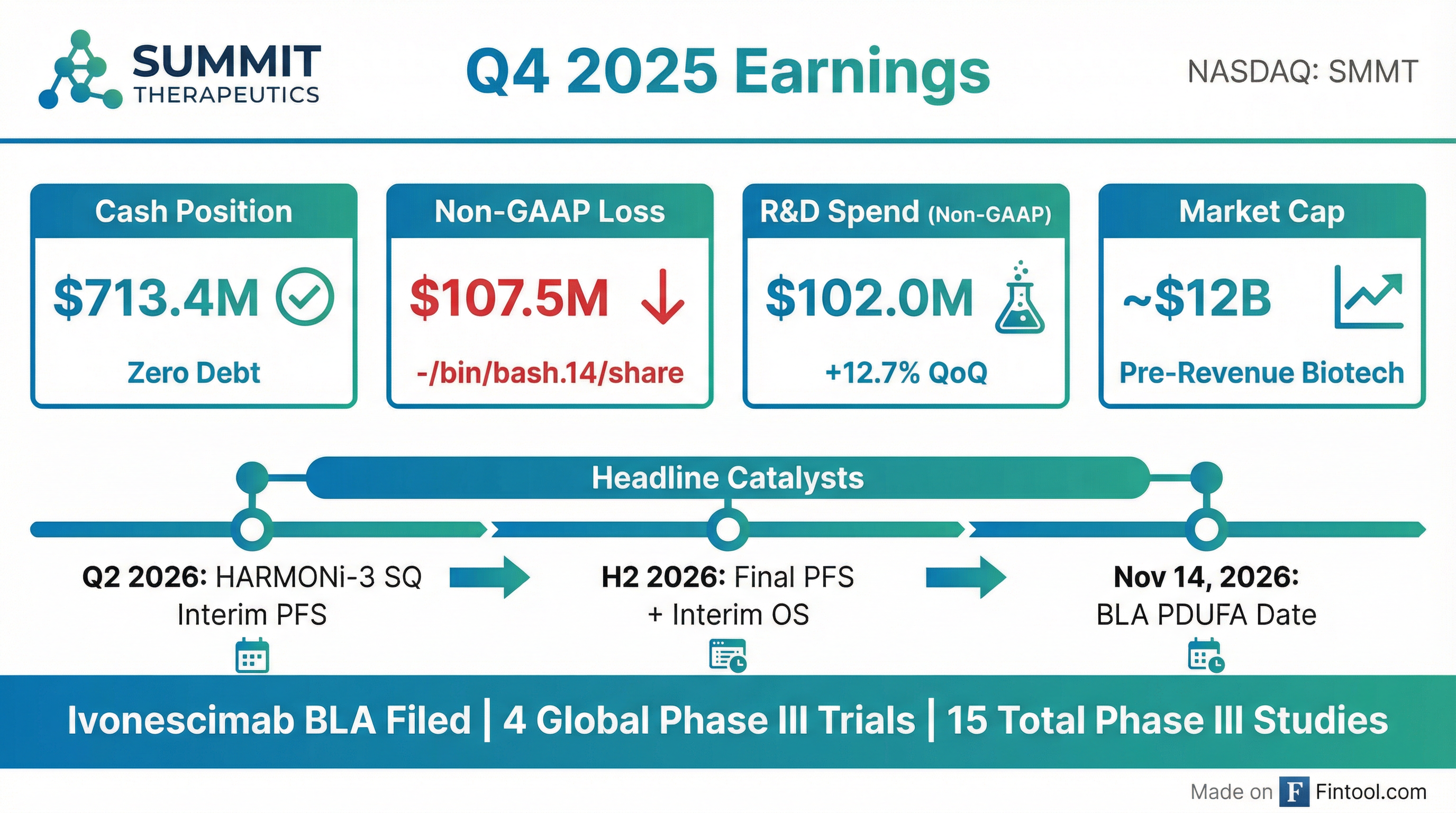
- Summit Therapeutics ended Q4 2025 with a strong cash position of approximately $713.4 million and no debt.
- The company announced an interim PFS analysis for the squamous cohort of the HARMONi-3 study is planned for Q2 2026, with final PFS and interim OS data expected in H2 2026.
- The US FDA accepted the Biologics License Application (BLA) for ivonesimab, setting a PDUFA target action date of November 14, 2026, for EGFR mutant non-small cell lung cancer.
- Total GAAP operating expenses for Q4 2025 were $225 million, while non-GAAP operating expenses were $113.3 million, primarily due to increased R&D for HARMONi-3 and HARMONi-7 trials.
- Summit is expanding its clinical program with a new Phase III ILLUMINE study in head and neck cancer, with initial enrollment expected early next quarter , and ongoing collaborations with Revolution Medicines and GSK.
- Summit Therapeutics concluded 2025 with a cash position of approximately $713.4 million and reported Q4 2025 GAAP operating expenses of $225 million.
- The company completed screening for the squamous cohort of the HARMONi-3 study and anticipates an interim PFS analysis during Q2 2026. Additionally, the BLA for ivonesimab in EGFR mutant non-small cell lung cancer was accepted by the FDA, with a PDUFA target action date of November 14, 2026.
- Ivonesimab has demonstrated positive data in all four Phase III clinical studies read out to date, resulting in two approvals in China, and currently has 15 Phase III trials announced, ongoing, or read out across various tumor types.
- New collaborations include a clinical trial with Revolution Medicines for RAS(ON) inhibitors, which has dosed its first patient, and a study with GSK for their B7-H3 antibody-drug conjugate, expected to begin dosing in mid-2026.
- Summit Therapeutics ended 2025 with a strong cash position of approximately $713.4 million and no debt.
- The company's Biologics License Application (BLA) for Ivonescimab in eGFR mutant non-small cell lung cancer after TKI therapy has been accepted by the FDA, with a PDUFA target action date of November 14, 2026.
- For the HARMONi-3 study, screening for the squamous cohort was completed ahead of schedule, and an interim Progression-Free Survival (PFS) analysis for this cohort is planned for Q2 2026.
- Total GAAP operating expenses for Q4 2025 were $225 million, while non-GAAP operating expenses increased to $113.3 million from $103.4 million in Q3 2025, primarily due to increased R&D expenses for HARMONi-3 and HARMONi-7 trials.
- Summit Therapeutics reported a GAAP net loss of $1,079.6 million or $(1.44) per basic and diluted share for the full year ended December 31, 2025, compared to $221.3 million or $(0.31) per share for the full year 2024.
- The company's cash and cash equivalents and short-term investments increased to $713.4 million at December 31, 2025, from $412.3 million at December 31, 2024.
- The US FDA accepted the Biologics License Application (BLA) filing for ivonescimab, with a PDUFA goal action date of November 14, 2026.
- For the HARMONi-3 squamous cohort, an interim Progression-Free Survival (PFS) analysis is planned for Q2 2026, and final PFS and interim Overall Survival (OS) data are expected in the second half of 2026.
- Summit Therapeutics reported a GAAP net loss of $1,079.6 million or $(1.44) per basic and diluted share for the full year 2025, compared to $221.3 million or $(0.31) per basic and diluted share for the full year 2024.
- The company's cash and cash equivalents and short-term investments increased to $713.4 million at December 31, 2025, from $412.3 million at December 31, 2024.
- The U.S. FDA accepted Summit's Biologics License Application (BLA) for ivonescimab, setting a PDUFA goal action date of November 14, 2026, for EGFR-mutated non-squamous NSCLC.
- For the HARMONi-3 squamous cohort, enrollment screening was completed in the first quarter of 2026, with an interim PFS analysis planned for Q2 2026 and final PFS and interim OS data expected in the second half of 2026.
- The Phase III ILLUMINE Study in 1L PD-L1 positive R/M HNSCC is expected to initiate with the first patient in early Q2 2026, and new clinical trial collaborations with Revolution Medicines and GSK are underway for ivonescimab.
- On January 29, 2026, Summit Therapeutics Inc. announced the U.S. Food & Drug Administration (FDA) accepted for filing its Biologics License Application (BLA) for ivonescimab.
- The BLA seeks approval for ivonescimab in combination with chemotherapy for patients with EGFR-mutated locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) post-tyrosine kinase inhibitor (TKI) therapy.
- The FDA has assigned a Prescription Drug User Fee Act (PDUFA) goal action date of November 14, 2026.
- The BLA filing is based on the overall results of the Phase III HARMONi trial, and ivonescimab previously received Fast Track designation from the US FDA.
- Summit Therapeutics submitted a Biologics License Application (BLA) to the FDA in Q4 2025 for ivonescimab in second-line EGFR mutant non-small cell lung cancer, with a regulatory decision anticipated around the end of 2026.
- Ivonescimab has demonstrated four positive Phase III readouts and secured two approvals in China, while currently enrolling three global Phase III trials across non-small cell lung cancer and colorectal cancer indications.
- The company entered 2026 with a cash balance of approximately $710 million and is debt-free, supported by a $500 million capital raise in October.
- Key upcoming catalysts for 2026 include the completion of enrollment for the Harmony III squamous cohort in H1 2026, with progression-free survival (PFS) and interim overall survival (OS) data expected in H2 2026, and the launch of additional new Phase III studies.
- Summit Therapeutics submitted a Biologics License Application (BLA) for ivonescimab to the FDA in Q4 2025, with a regulatory decision anticipated around the end of 2026.
- The company reported a cash balance of approximately $710 million entering 2026 and remains debt-free, following a $500 million capital raise in October.
- Upcoming clinical milestones for ivonescimab include expected completion of enrollment for the squamous cohort of the HARMONi-3 trial in H1 2026, with progression-free survival and interim overall survival data in H2 2026.
- Ivonescimab has completed four positive Phase III studies and has received two approvals in China for non-small cell lung cancer indications.
- Summit Therapeutics announced new clinical collaborations for ivonescimab with Revolution Medicines (expected to begin dosing this quarter) and GSK (expected to start mid-2026).
- Summit Therapeutics' lead asset, ivonescimab, has completed four positive Phase III studies and is positioned as a potential first-mover in the PD-1/VEGF space, targeting a market estimated to exceed $100 billion annually.
- A Biologics License Application (BLA) for ivonescimab was submitted to the FDA in Q4 2025 for second-line EGFR mutant non-small cell lung cancer, with a regulatory decision anticipated around the end of 2026.
- The company is actively enrolling three global Phase III trials (Harmony III, Harmony VII, Harmony GI III), with key data readouts expected for Harmony III's squamous arm (PFS and interim OS in H2 2026) and non-squamous arm (PFS in H1 2027).
- Summit Therapeutics reported a cash balance of approximately $710 million entering 2026, following a $500 million capital raise in October 2025, and remains debt-free.
- Summit Therapeutics submitted a Biologics License Application (BLA) to the U.S. FDA in Q4 2025 for ivonescimab in combination with chemotherapy for the second-line or later treatment of patients with EGFR-mutated non-small cell lung cancer (NSCLC). A decision from the FDA is anticipated by the fourth quarter of 2026.
- As of December 31, 2025, the company reported a preliminary unaudited balance of approximately $710 million in cash, cash equivalents, and short-term investments, with no debt.
- The company announced a clinical trial collaboration with GSK to evaluate ivonescimab in combination with GSK's B7-H3 antibody drug conjugate (ADC), risvutatug rezetecan, across multiple solid tumor settings, with clinical trials expected to begin in mid-2026.
Quarterly earnings call transcripts for Summit Therapeutics.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more