Earnings summaries and quarterly performance for Tarsus Pharmaceuticals.
Executive leadership at Tarsus Pharmaceuticals.
Bobak Azamian
Chief Executive Officer
Aziz Mottiwala
Chief Commercial Officer
Bryan Wahl
General Counsel and Secretary
Dianne Whitfield
Chief Human Resources Officer
Elizabeth Yeu-Lin
Chief Medical Officer
Jeffrey Farrow
Chief Financial Officer and Chief Strategy Officer
Seshadri Neervannan
Chief Operating Officer
Board of directors at Tarsus Pharmaceuticals.
Research analysts who have asked questions during Tarsus Pharmaceuticals earnings calls.
Eddie Hickman
Guggenheim Securities
6 questions for TARS
Lachlan Hanbury-Brown
William Blair & Company
6 questions for TARS
Cory Jubinville
LifeSci Capital
4 questions for TARS
Andrea Newkirk
Goldman Sachs
3 questions for TARS
Jason Gerberry
Bank of America Merrill Lynch
3 questions for TARS
Matthew Caufield
H.C. Wainwright & Co., LLC
3 questions for TARS
Pavan Patel
Bank of America
3 questions for TARS
Andreas Argyrides
Oppenheimer & Co.
2 questions for TARS
Anthea Li
Jefferies
2 questions for TARS
François Brisebois
Oppenheimer & Co. Inc.
2 questions for TARS
Graig Suvannavejh
Mizuho Securities
2 questions for TARS
Jena Davidner
Barclays
2 questions for TARS
Oren Livnat
H.C. Wainwright
2 questions for TARS
Andreas Argyrides
Oppenheimer & Co. Inc.
1 question for TARS
Andrea Tan
Goldman Sachs
1 question for TARS
Balaji Prasad
Barclays
1 question for TARS
Recent press releases and 8-K filings for TARS.
- Tarsus Pharmaceuticals reported over $450 million in revenue for XDEMVY in its second year of launch and projects $2 billion+ in peak sales. The company issued its first annual sales guidance of $670 million to $700 million for the full year, representing approximately 50% growth at the midpoint.
- XDEMVY has treated half a million patients out of a 25 million patient opportunity for Demodex blepharitis, with 90% payer coverage achieved. Tarsus invested $80 million in direct-to-consumer advertising in 2025 to raise disease awareness to 25% and is expanding its sales force to deepen prescriber engagement.
- The company is advancing TP-04 for Ocular Rosacea (Phase 2, data expected H1 2027, targeting 15-18 million patients) and TP-05 for Lyme disease prevention (Phase 2, data expected H1 2027, with a goal for a Phase 3 ready partner package).
- While clinical trials showed a 40% recurrence rate for XDEMVY, the company conservatively models 20% for refills, with current real-world data approaching this figure. Tarsus expects typical seasonality, with Q1 sales projected to be flat to slightly down from Q4 due to deductible resets.
- Tarsus Pharmaceuticals reported over $450 million in revenue for XDEMVY in its second year of launch, projecting $2 billion+ in peak sales for the drug, which has already treated half a million patients and achieved 90% payer coverage.
- The company provided its first sales guidance, expecting $670 million - $700 million for full-year 2026, with Q1 2026 sales anticipated to be flat to slightly down compared to Q4 2025 due to typical seasonality.
- Tarsus is advancing its pipeline with TP-04 for Ocular Rosacea in a Phase 2 study, with data expected in the first half of 2027, and TP-05 for Lyme disease prevention also in a Phase 2 study, targeting proof of concept data in the first half of 2027 for a potential partnership.
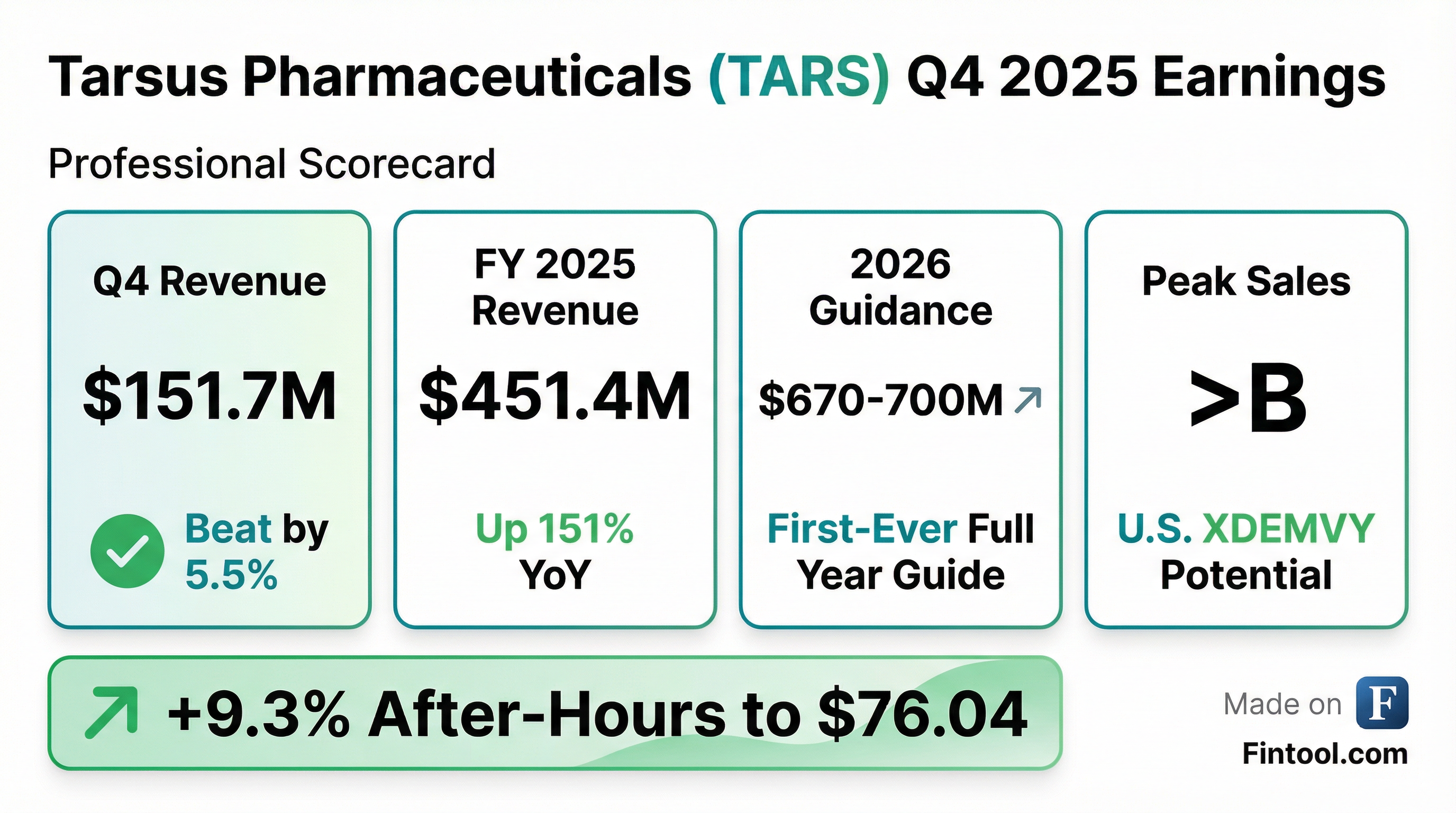
- Tarsus Pharmaceuticals reported full-year 2025 net sales of $451.4 million and Q4 2025 net product sales of $151.7 million.
- The company provided full-year 2026 net product sales guidance in the range of $670 million-$700 million, representing over 50% growth at the midpoint.
- Tarsus now believes XDEMVY can reach blockbuster status within the next couple of years, with sales potential exceeding $2 billion in the U.S..
- The pipeline is advancing with a Phase 2 trial for TP-04 in ocular rosacea initiated in December 2025 and a Phase 2 clinical trial for TP-05 in Lyme disease prevention planned for Q2 2026.
- The company ended 2025 with approximately $418 million in cash, cash equivalents, and marketable securities.
- Tarsus Pharmaceuticals reported $151.7 million in net product sales for Q4 2025 and $451.4 million for the full year 2025.
- The company provided full-year 2026 net product sales guidance of $670 million-$700 million, representing over 50% annual growth at the midpoint.
- Tarsus updated the peak sales potential for XDEMVY to exceed $2 billion, having already helped over 500,000 patients since launch.
- Operating expenses for 2025 were $522.3 million, and 2026 guidance includes SG&A expenses of $545 million-$565 million and R&D expenses of $115 million-$135 million.
- The pipeline advanced with the initiation of a Phase II trial for TP-04 in ocular rosacea in December 2025 and planned initiation of a Phase II trial for TP-05 in Lyme disease prevention in Q2 2026, both with top-line data expected in the first half of 2027.
- Tarsus Pharmaceuticals reported Q4 2025 XDEMVY net sales of $151.7 million, a 128% year-over-year increase, and full-year 2025 XDEMVY net sales of $451.4 million, up 150% year-over-year.
- For 2026, the company forecasts XDEMVY net sales of $670 million to $700 million, with a gross margin of approximately 93%.
- The Phase 2 trial for TP-04 (Ocular Rosacea) was initiated in December 2025, with data expected in 1H 2027.
- A Phase 2 trial for TP-05 (Lyme Disease Prevention) is planned to commence in Q2 2026.
- The company is advancing global regulatory pathways for TP-03 (Demodex blepharitis), targeting potential approval in Greater China in 2026 and Europe in 2027.
- Tarsus Pharmaceuticals reported full-year 2025 net sales exceeding $450 million for XDEMVY and provided full-year 2026 net sales guidance of $370 million to $400 million.
- The company raised the peak sales potential for XDEMVY to over $2 billion.
- Tarsus initiated a Phase 2 trial for TP-04 in ocular rosacea in December 2025 and plans to initiate a Phase 2 clinical trial for TP-05 in Lyme disease prevention in Q2 2026, with top-line data for both expected in the first half of 2027.
- To support growth, the company plans to add approximately 15-20 key account leaders to its sales force in 2026.
- David Pyott, former chairman and CEO of Allergan, joined the board of directors.
- Tarsus Pharmaceuticals (TARS) reported full-year 2025 net product sales of XDEMVY® of $451.4 million, an increase of over 150% year-over-year, and Q4 2025 net product sales of $151.7 million.
- The company projects XDEMVY peak sales potential of more than $2 billion.
- For full-year 2025, Tarsus reported a net loss of $66.4 million and net loss per share of $(1.59), improving from a net loss of $115.6 million and net loss per share of $(3.07) in 2024. For Q4 2025, the net loss was $8.4 million and net loss per share was $(0.20).
- Tarsus is advancing its pipeline with the initiation of a Phase 2 trial of TP-04 in ocular rosacea and plans to initiate a Phase 2 trial of TP-05 for Lyme disease prevention in Q2 2026.
- Tarsus Pharmaceuticals, Inc. expects Q4 2025 net sales for XDEMVY to be between $140 million and $145 million, contributing to an estimated FY 2025 net sales of $440 million to $445 million. The company projects XDEMVY peak sales potential to exceed $1 billion beyond 2026.
- As of December 2025, over 500,000 patients have been treated with XDEMVY since launch, with more than 11,000 weekly prescriptions and over 20,000 Eye Care Professionals (ECPs) prescribing the treatment. The company reported Q3 2025 net sales of $118.7 million, representing a 147% year-over-year growth.
- The company initiated a Phase 2 study for TP-04 (ocular rosacea) in December 2025, with data expected in 1H 2027. Tarsus also plans to initiate a Phase 2 study for TP-05 (Lyme disease prevention) in 2026 and is pursuing global expansion for XDEMVY, including potential regulatory approval in Europe by 2027 and ongoing discussions in Japan.
- Tarsus Pharmaceuticals reported strong Q3 2025 revenue of almost $119 million for XDEMVY and provided Q4 2025 revenue guidance of $140-$145 million, projecting full-year 2025 revenues between $440-$445 million.
- The company adjusted its long-term gross-to-net discount expectation to 43%-45% due to a higher mix of Medicare patients.
- Tarsus is progressing its pipeline, with a Phase 2 study for ocular rosacea (TP-04) commencing by year-end 2025 and top-line data anticipated in H2 2026.
- The company is well-capitalized, holding approximately $400 million at the end of Q3 2025 to support its commercial product and pipeline development.
- Tarsus reported XDEMVY net sales of $118.7 million for Q3 2025, marking approximately 147% year-over-year growth.
- The company delivered over 103,000 XDEMVY bottles to patients in Q3 2025.
- For Q4 2025, Tarsus anticipates XDEMVY net product sales to be between $140 million and $145 million, with full-year 2025 XDEMVY net product sales projected to range from $440 million to $445 million.
- Tarsus plans to initiate a Phase 2 study for TP-04 (ocular rosacea) in Q4 2025 and a Phase 2 study for TP-05 (Lyme disease prevention) in 2026, while also progressing towards a potential submission in Europe in 2026 and having a New Drug Application accepted in China for TP-03.
Quarterly earnings call transcripts for Tarsus Pharmaceuticals.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more