Earnings summaries and quarterly performance for Tonix Pharmaceuticals Holding.
Executive leadership at Tonix Pharmaceuticals Holding.
Board of directors at Tonix Pharmaceuticals Holding.
Research analysts covering Tonix Pharmaceuticals Holding.
Recent press releases and 8-K filings for TNXP.
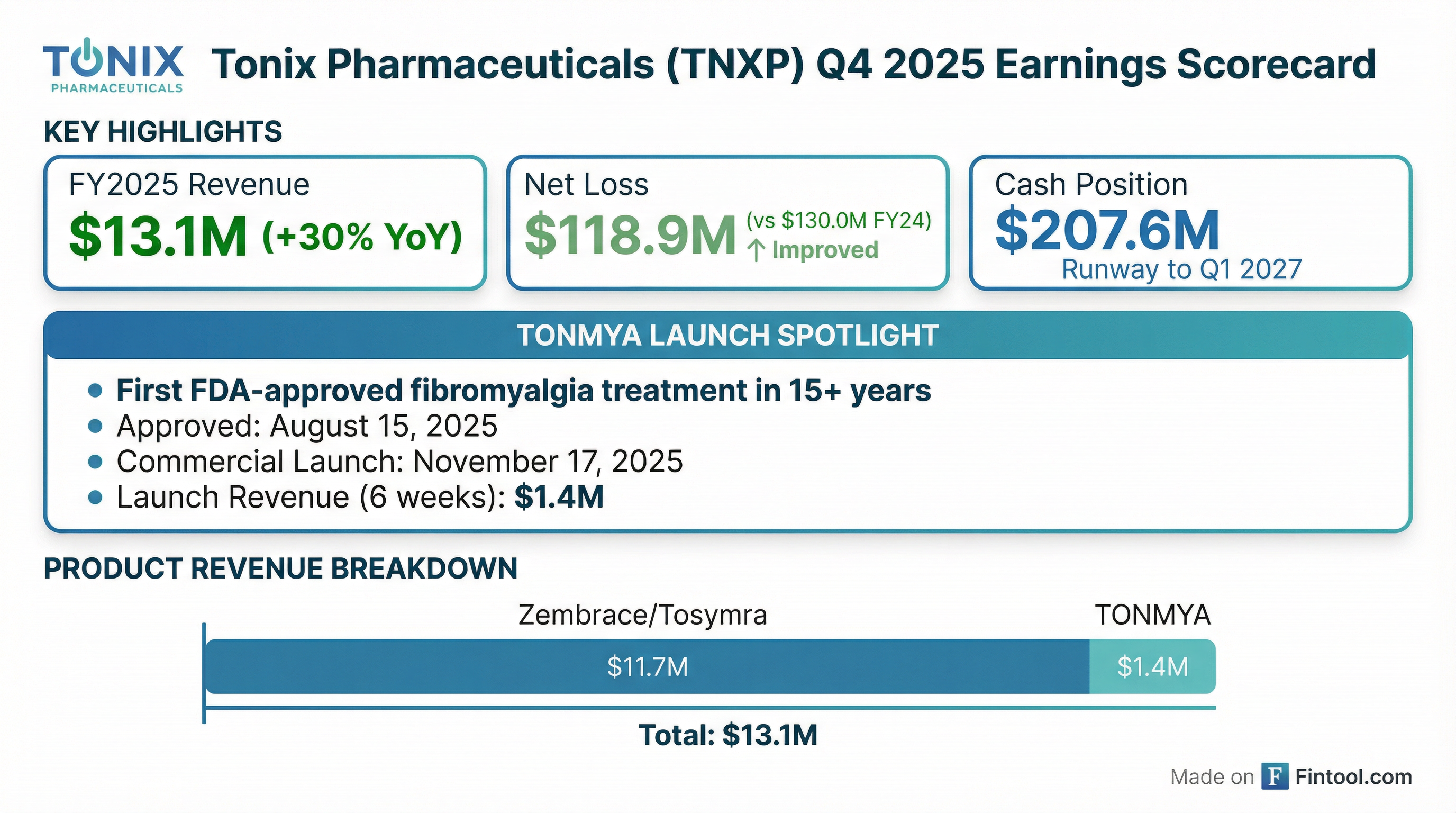
Tonix Pharmaceuticals reports preliminary Q4 and FY 2025 financial results
TNXP
Earnings
Guidance Update
Revenue Acceleration/Inflection
- Tonix Pharmaceuticals Holding Corp. reported preliminary net revenue of approximately $13.1 million for the year ended December 31, 2025, an increase from $10.1 million in the prior year.
- The company's preliminary net loss for the year ended December 31, 2025, was approximately $118.9 million, compared to $130.0 million for the year ended December 31, 2024.
- Net cash used in operating activities for the year ended December 31, 2025, was approximately $99.0 million, an increase from $60.9 million in the previous year.
- As of December 31, 2025, the company held approximately $207.6 million in cash and cash equivalents, which are anticipated to meet operating and capital expenditure requirements into the first quarter of 2027.
- These financial results for the quarter and year ended December 31, 2025, are preliminary and subject to completion and potential material adjustments.
4 days ago
Tonix Pharmaceuticals Highlights Positive Phase 3 Data for FDA-Approved TONMYA™
TNXP
Product Launch
- Tonix Pharmaceuticals presented positive Phase 3 RESILIENT study data for TONMYA™ (sublingual cyclobenzaprine HCl) at the 2026 Non-Opioid Pain Therapeutics Summit.
- The study demonstrated a statistically significant reduction in weekly average pain scores at Week 14 (p<0.0001) versus placebo, with the treatment being well tolerated and minimal effects on weight or blood pressure.
- TONMYA™ was approved by the FDA on August 15, 2025, for the treatment of fibromyalgia in adults, making it the first new prescription medicine approved for fibromyalgia in over 15 years.
- Its unique sublingual formulation is designed for bedtime administration to optimize parent-drug exposure during sleep and decrease active metabolite levels, addressing non-restorative sleep central to fibromyalgia.
8 days ago
Tonix Pharmaceuticals Announces $20.0 Million Registered Direct Offering
TNXP
New Projects/Investments
- Tonix Pharmaceuticals Holding Corp. (TNXP) announced a registered direct offering of 615,025 shares of common stock at $16.26 per share and pre-funded warrants to purchase up to 615,025 shares of common stock at $16.259 per pre-funded warrant.
- The offering is expected to generate approximately $20.0 million in gross proceeds before deducting placement agent fees and other estimated offering expenses, with the closing anticipated on or about December 30, 2025.
- The net proceeds from the offering will be used to fund the commercialization of its marketed products, the development of its product pipeline, and for general working capital and corporate purposes.
- TD Securities (USA) LLC, also referred to as TD Cowen, is acting as the sole placement agent for the offering, with a placement agency fee totaling $1,200,036.78.
Dec 29, 2025, 9:44 PM
Tonix Pharmaceuticals Licenses TNX-4900 for Chronic Neuropathic Pain
TNXP
New Projects/Investments
- Tonix Pharmaceuticals Holding Corp. announced on December 16, 2025, that it licensed exclusive worldwide rights to TNX-4900 (formerly PW507) from Rutgers University.
- TNX-4900 is a highly selective small-molecule Sigma-1 receptor (S1R) antagonist being developed for chronic neuropathic pain.
- This non-opioid analgesic has shown efficacy in multiple animal pain models, including diabetic and chemotherapy-induced neuropathic pain, and exhibits a compelling safety and pharmacokinetic profile.
- Tonix plans to advance TNX-4900 through expanded pharmacokinetic, formulation, and safety studies to support IND-enabling development.
Dec 16, 2025, 12:05 PM
Tonix Pharmaceuticals Launches TONMYA for Fibromyalgia
TNXP
Product Launch
- Tonix Pharmaceuticals Holding Corp. announced the commercial launch of TONMYA™ (cyclobenzaprine HCl sublingual tablets) in the U.S. on November 17, 2025, making it available by prescription nationwide.
- TONMYA is a first-in-class, non-opioid, once-daily bedtime analgesic for fibromyalgia, representing the first FDA-approved treatment for the condition in over 15 years.
- The drug received FDA approval on August 15, 2025, and targets an estimated 10 million adults in the U.S. suffering from fibromyalgia.
- The company expects U.S. market exclusivity for TONMYA until 2034, with potential extensions to 2044 based on pending patent applications.
Nov 17, 2025, 12:05 PM
Tonix Provides Update on Tonmya Approval, Launch, and Pipeline
TNXP
Product Launch
New Projects/Investments
Guidance Update
- Tonix (TNXP) received FDA approval for Tonmya for fibromyalgia on August 15, with a commercial launch in the United States scheduled before the end of November.
- Tonmya is the first product approved for fibromyalgia in over 15 years, addressing a market of 10 million American adults, of whom 3 million are diagnosed and treated.
- The company reported $190 million in cash at the end of September, with no debt, and anticipates a cash runway into the first quarter of 2027, which fully funds the launch.
- Tonix is advancing several pipeline programs, including TNX-102 SL for acute stress disorder and major depressive disorder, an anti-CD40 ligand for allograft rejection (Phase 2 starting H1 next year), and TNX-4800, a phase 2 ready preventative for Lyme disease.
Nov 13, 2025, 3:00 PM
Tonix Pharmaceuticals Reports Q3 2025 Financial Results and Tonmya Launch
TNXP
Earnings
Product Launch
New Projects/Investments
- Tonix Pharmaceuticals reported $190.1 million in cash and cash equivalents as of September 30, 2025, with a cash runway projected to fund operations into the first quarter of 2027.
- The company is set to launch Tonmya™, the first new FDA-approved medicine for fibromyalgia in over 15 years, in November 2025 following its August approval.
- For the third quarter ended September 30, 2025, Tonix reported net product revenue of $3.3 million and a net loss of $32.0 million, or $3.59 per share.
- Tonix is advancing its pipeline with plans to initiate a Phase 2 study for TNX-102 SL (MDD) mid-2026, a Phase 2 study for TNX-1500 (kidney transplant rejection) in 1H 2026, and an adaptive Phase 2/3 study for TNX-4800 (Lyme disease prevention) in 2027.
Nov 10, 2025, 9:15 PM
Tonix Pharmaceuticals Holding Corp. Announces Preliminary Q3 2025 Results and Tonmya™ Launch
TNXP
Earnings
Product Launch
Guidance Update
- Tonix Pharmaceuticals Holding Corp. reported preliminary Q3 2025 results, including a net loss of approximately $32.0 million and net revenue of approximately $3.3 million from marketed products.
- The company ended Q3 2025 with approximately $190.1 million in cash and cash equivalents and anticipates its cash resources, along with $29.3 million from Q4 2025 equity offerings, will fund operations into Q1 2027.
- The U.S. commercial launch of Tonmya™ for the treatment of fibromyalgia in adults is expected by the end of November 2025.
Oct 27, 2025, 11:00 AM
Tonix Pharmaceuticals Presents Positive Tonmya Data and Highlights Recent FDA Approval
TNXP
Product Launch
New Projects/Investments
- Tonix Pharmaceuticals presented data on Tonmya (TNX-102 SL) at the 2025 American College of Rheumatology (ACR) Convergence, demonstrating significant reduction in fibromyalgia pain compared with placebo in the Phase 3 RESILIENT study.
- The treatment was well tolerated with minimal effects on weight or blood pressure, and a low rate of adverse event-related discontinuations.
- Tonmya was approved by the FDA on August 15, 2025, for the treatment of fibromyalgia in adults, representing the first approval for a new prescription medicine for fibromyalgia in more than 15 years.
Oct 27, 2025, 11:00 AM
Tonix Pharmaceuticals Presents Positive Preclinical Data for TNX-801 Mpox Vaccine
TNXP
New Projects/Investments
- Tonix Pharmaceuticals presented positive preclinical data for its TNX-801 mpox and smallpox vaccine candidate at the World Vaccine Congress–Europe 2025, held from October 14–16, 2025.
- The data demonstrated favorable safety, immunogenicity, and durable protection for at least 14 months post-vaccination across multiple preclinical models.
- These results support the advancement of TNX-801 toward clinical development, with Tonix planning a Phase I clinical study in Kenya through a collaboration with the Kenya Medical Research Institute (KEMRI).
Oct 17, 2025, 11:00 AM
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more