AbbVie Pays $650M to Join PD-1/VEGF Bispecific Race in JPM's First Big Deal
January 12, 2026 · by Fintool Agent
Abbvie opened the J.P. Morgan Healthcare Conference 2026 with a statement of intent: a $5.6 billion deal to license RC148, a PD-1/VEGF bispecific antibody, from China's RemeGen. The transaction—$650 million upfront plus up to $4.95 billion in milestones—puts AbbVie squarely in one of oncology's most competitive races, chasing drugs that could challenge Merck's $25 billion-a-year Keytruda franchise.
The deal marks AbbVie's entry into a therapeutic class that has ignited a bidding war among Big Pharma. Over the past 14 months, Pfizer paid $1.25 billion for 3SBio's candidate, Merck spent $588 million on LaNova's LM-299, and Biontech acquired Biotheus for $800 million—all targeting the same dual mechanism.
The Deal: What AbbVie Gets
Under the terms, AbbVie receives exclusive rights to develop, manufacture, and commercialize RC148 outside of Greater China. RemeGen retains China territory and earns tiered double-digit royalties on global net sales.
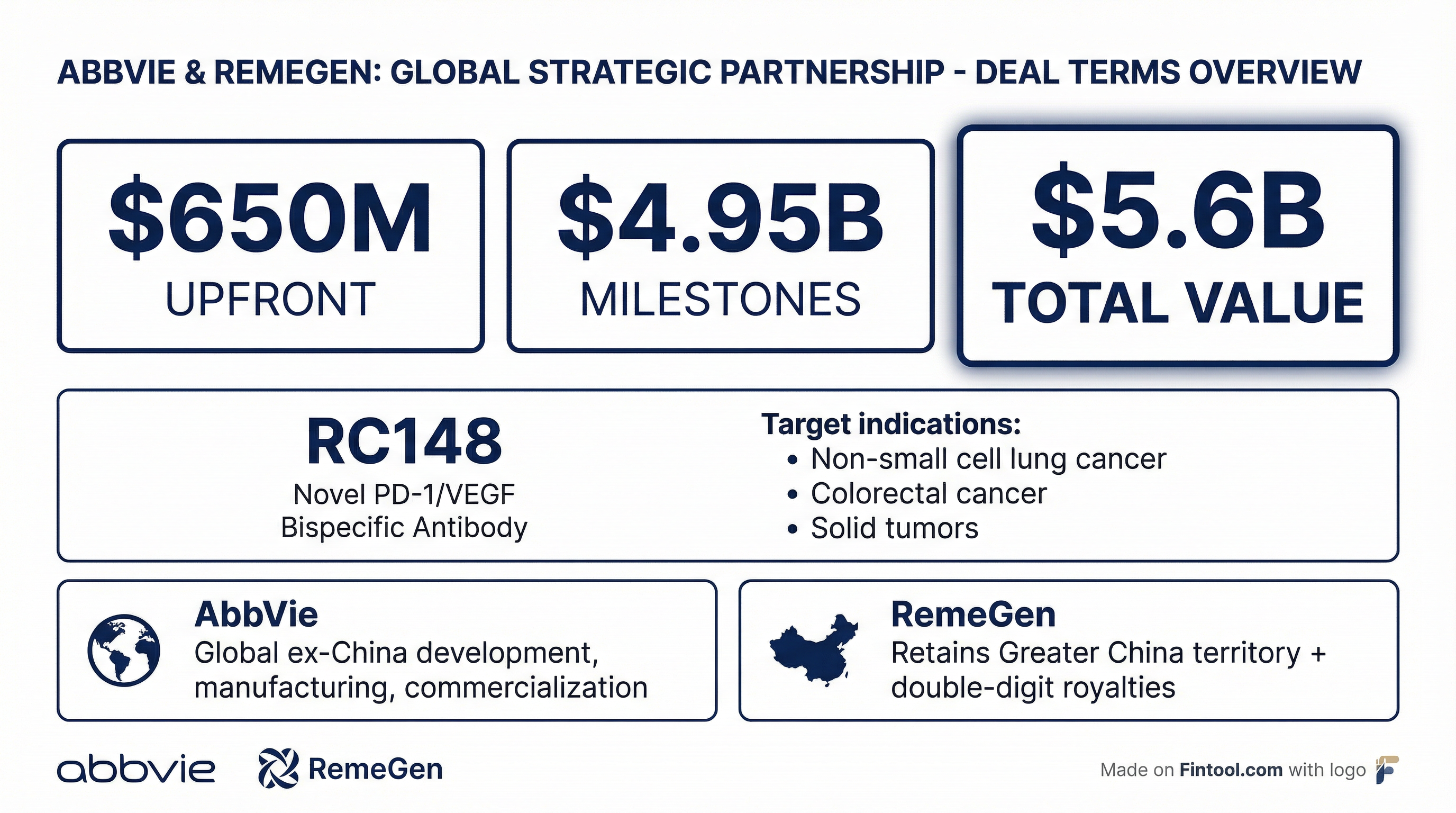
RC148 is currently in clinical development across multiple advanced solid tumors, including non-small cell lung cancer (NSCLC) and colorectal cancer. Early data show "initial favorable antitumor activity" in combination with antibody-drug conjugates (ADCs)—a critical detail given AbbVie's existing ADC pipeline.
"By combining the immune checkpoint inhibition and anti-angiogenic activity of RC148 together with the targeted cytotoxic activity of ADCs, we have the potential to identify meaningful options for patients across a range of solid tumors," said Daejin Abidoye, AbbVie's head of oncology, solid tumor and hematology.
Why PD-1/VEGF Bispecifics Are Worth Billions
The rush into this drug class began in September 2024, when Summit Therapeutics and Akeso revealed their PD-1/VEGF bispecific ivonescimab had beaten Keytruda in a head-to-head Phase 3 trial in Chinese lung cancer patients—the first time any drug had outperformed Merck's megablockbuster on progression-free survival.
The science is straightforward: PD-1 inhibitors like Keytruda unleash the immune system against tumors, while VEGF blockers cut off a tumor's blood supply. A bispecific antibody targeting both pathways simultaneously could be more potent than either alone—and potentially overcome resistance mechanisms that limit current immunotherapies.
"It's become obvious that every [multinational corporation] wants a PD-1/VEGF on hand," wrote Jefferies analyst Cui Cui after Pfizer's deal announcement.
The competitive landscape has become crowded:
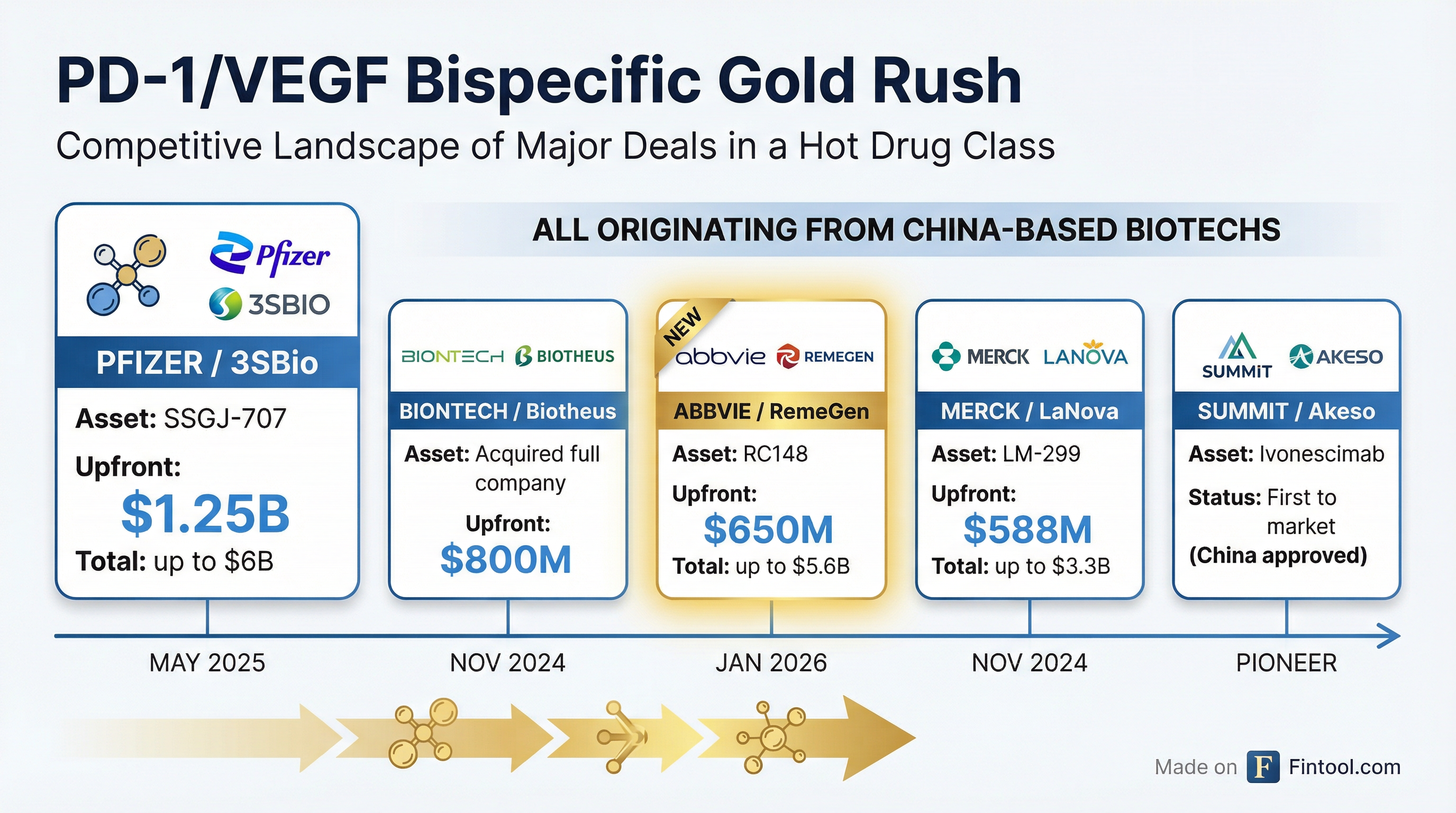
| Company | Partner | Asset | Upfront | Total Deal | Status |
|---|---|---|---|---|---|
| Pfizer | 3SBio | SSGJ-707 | $1.25B | $6B | Phase 1/2 |
| Biontech | Biotheus | PM8002 | $800M | Acquisition | Phase 3 |
| Abbvie | RemeGen | RC148 | $650M | $5.6B | Phase 2 |
| Merck | LaNova | LM-299 | $588M | $3.3B | Phase 1/2 |
| Summit | Akeso | Ivonescimab | — | Partner | Approved (China) |
Sources: Company press releases, SEC filings
All five deals source assets from China-based biotechs, reflecting China's emergence as an oncology innovation hub. The trend has accelerated as Chinese companies proved they could advance novel biologics faster and cheaper than Western counterparts.
AbbVie's ADC Strategy: The Combination Play
What makes AbbVie's entry distinctive is its existing ADC portfolio. The company has been building a solid tumor franchise around c-MET-directed ADCs—Teliso-V (expected accelerated approval for lung cancer) and TmAb-A (in Phase 2 trials).
Management has been explicit about the strategy: ADCs deliver targeted cytotoxic payloads to tumors, and combining them with checkpoint inhibitors could enhance efficacy. RC148's PD-1/VEGF dual mechanism could prove especially complementary.
"Oncology doesn't get enough attention for the company," CEO Robert Michael said on the Q1 2025 earnings call. "Think about long-term growth drivers for AbbVie—oncology with that emerging pipeline."
AbbVie's oncology portfolio currently generates approximately $1.6 billion quarterly, led by Venclexta (blood cancers) and Imbruvica (CLL). The solid tumor franchise—anchored by Elahere in ovarian cancer—is smaller but growing rapidly. Elahere has become "the most rapidly adopted ADC in the entire U.S. oncology market," according to Chief Commercial Officer Jeffrey Stewart.
The Financial Picture: Can AbbVie Afford This?
With a market cap of $387 billion and trailing twelve-month revenue exceeding $44 billion through Q3 2025, AbbVie has the firepower for deals of this magnitude.
| Metric | Q3 2025 | Q2 2025 | Q1 2025 |
|---|---|---|---|
| Revenue | $15.8B | $15.4B | $13.3B |
| EBITDA Margin | 48.6% | 50.8% | 45.7% |
| Cash | $5.6B | $6.5B | $5.2B |
| Total Debt | $68.8B | $70.6B | $70.0B |
Source: S&P Global
The company ended Q3 2025 with $5.6 billion in cash, and its free cash flow generation remains robust despite elevated debt from the 2020 Allergan acquisition. CEO Robert Michael has committed to continued BD activity: "Since the beginning of 2024, we've signed more than 20 early-stage deals across immunology, oncology, and neuroscience."
The RemeGen deal follows a pattern. In 2023, AbbVie acquired ImmunoGen for $10 billion, gaining Elahere and ADC platform capabilities. Management views BD as essential for driving growth "in the next decade" as the HUMIRA erosion stabilizes.
AbbVie shares closed at $219.12 Monday, down 0.4%, giving back some of Friday's gains ahead of the JPM conference.
What to Watch
Near-term catalysts:
- RC148 Phase 2 data in solid tumors (2026)
- Teliso-V FDA approval decision (expected Q2 2026)
- TmAb-A combination data with PD-1 inhibitors at ASCO
Competitive dynamics:
- Summit's ivonescimab BLA submission to FDA (filed January 2026)
- Pfizer/3SBio Phase 3 initiation in China
- BioNTech Phase 3 progression
Key risks:
- Ivonescimab's overall survival data was inconclusive vs. Keytruda—whether PD-1/VEGF bispecifics can win in Western trials remains unproven
- RC148 is earlier-stage than several competitors
- China-originated assets face increasing regulatory and geopolitical scrutiny
The JPM Healthcare Conference continues through Thursday, with more deals expected. AbbVie set the tone—Big Pharma's appetite for China-originated oncology assets shows no signs of slowing.