Bristol Myers Bets $850M on Janux's 'Masked' Cancer Drugs
January 22, 2026 · by Fintool Agent
Bristol Myers Squibb is paying $50 million upfront to license a novel tumor-activated cancer therapy from Janux Therapeutics, with the deal potentially worth up to $850 million including development and commercial milestones. JANX shares surged 14% to $15.14 on the news, adding roughly $115 million in market value to the small-cap biotech.
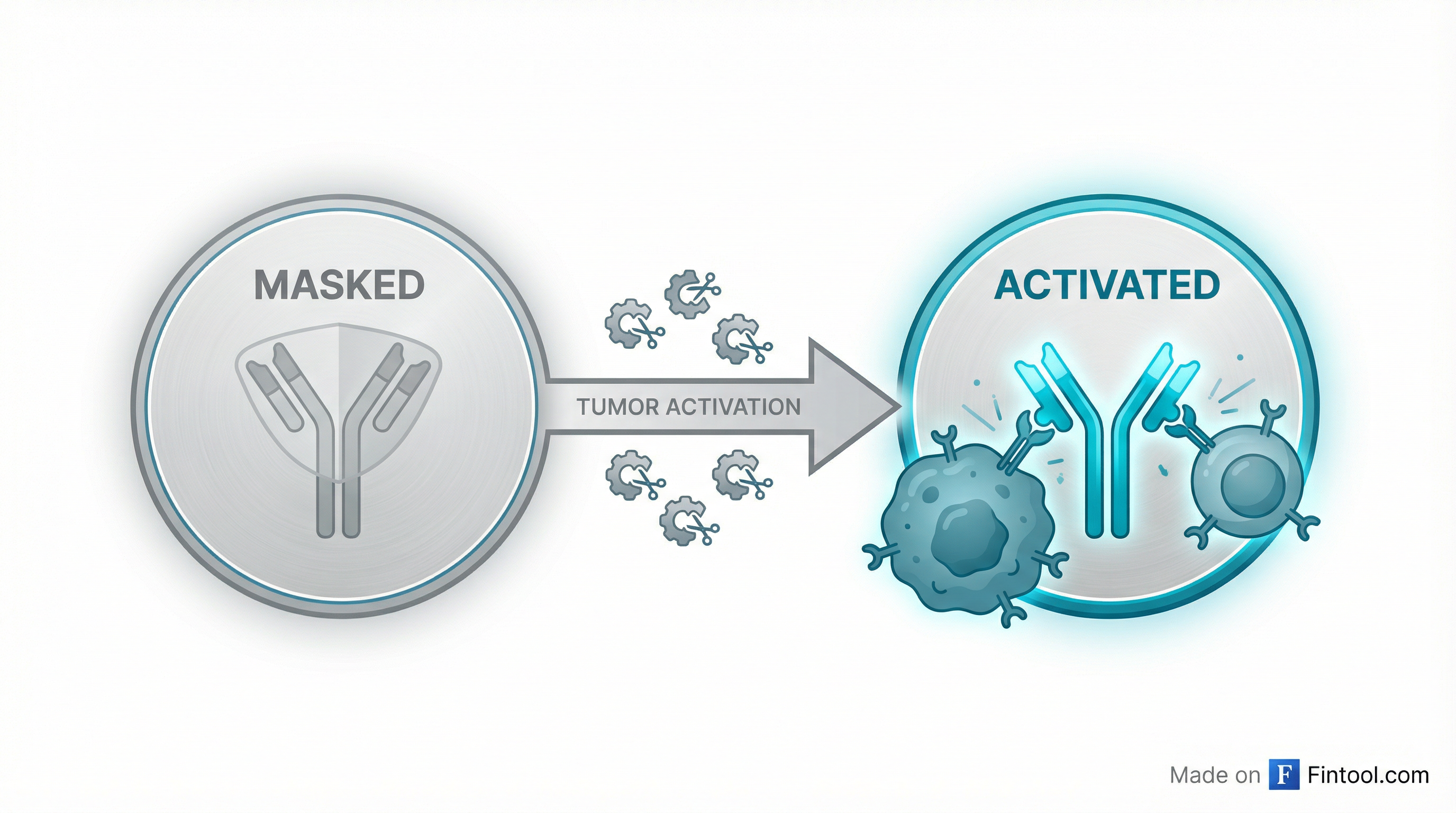
The deal validates Janux's proprietary TRACTr platform—a technology designed to solve a fundamental problem that has derailed multiple T-cell engager programs: the drugs work, but they're too toxic. By "masking" the T-cell binding site until the drug reaches the tumor, Janux aims to avoid the systemic cytokine storms that have killed programs from Amgen, Johnson & Johnson, and others.
"This collaboration marks a significant milestone for Janux, validating the strength of our tumor-activated platforms and expanding our reach in solid tumor oncology," said David Campbell, Ph.D., President and CEO of Janux.
Deal Structure
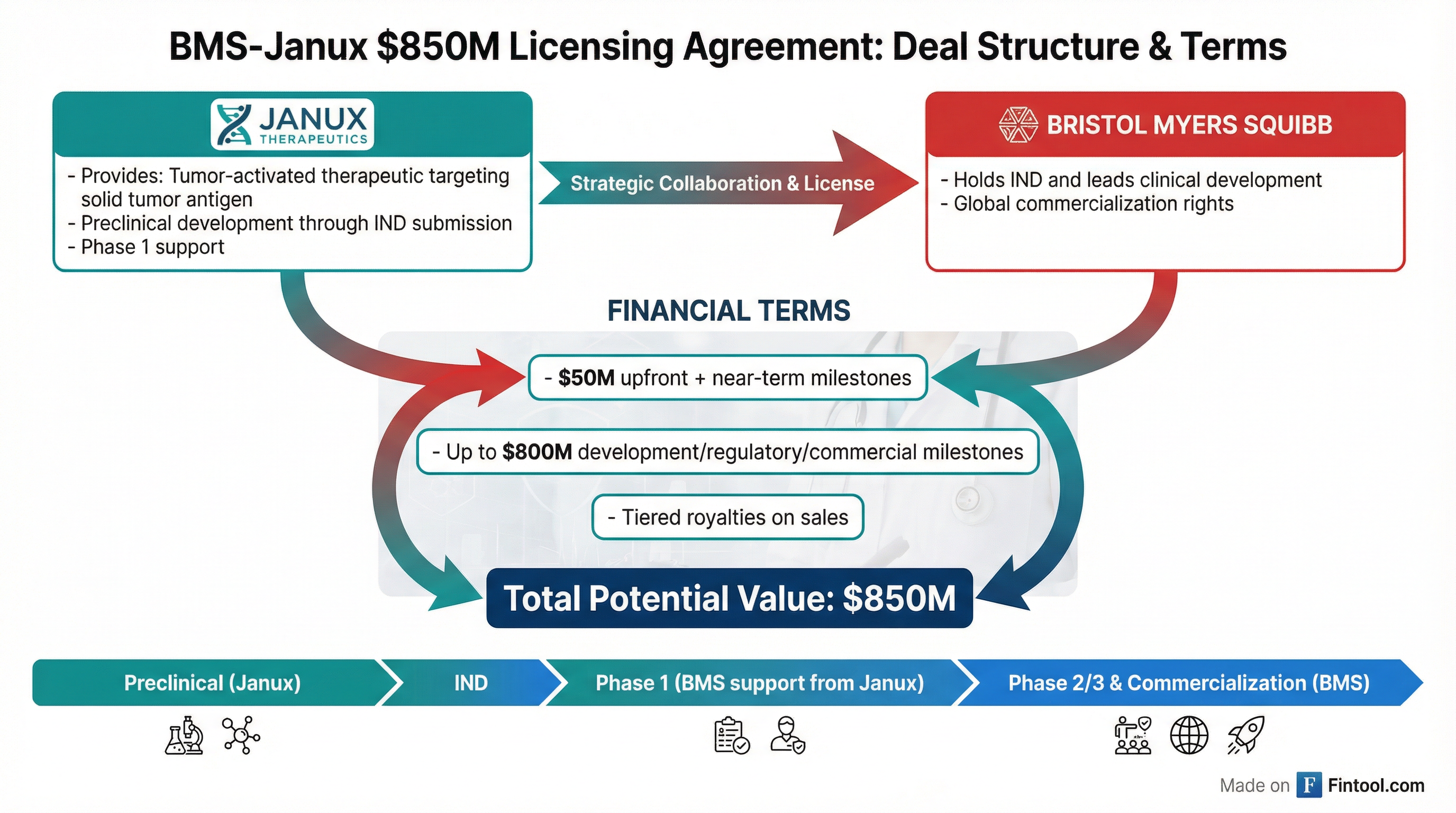
Under the agreement, Janux will complete preclinical development through IND submission for an undisclosed therapeutic targeting "a validated solid tumor antigen expressed across several human cancer types." Bristol Myers will hold the IND and lead clinical development and global commercialization, with Janux providing support through completion of the first Phase 1 study.
| Component | Value |
|---|---|
| Upfront + Near-Term Milestones | Up to $50M |
| Development, Regulatory & Commercial Milestones | Up to $800M |
| Royalties | Tiered on global sales |
| Total Potential Value | ~$850M |
Why T-Cell Engagers Keep Failing in Solid Tumors
T-cell engagers have transformed treatment of blood cancers—Amgen's Blincyto for leukemia, J&J's Tecvayli for multiple myeloma. But solid tumors have been a graveyard for the technology.
The problem is on-target, off-tumor toxicity. These bispecific drugs grab both a cancer-associated antigen and the CD3 receptor on T-cells, forcing an immune attack. But when the target antigen exists on healthy tissue—even at low levels—the resulting cytokine release can be severe. Programs from Amgen (AMG160, AMG212), Johnson & Johnson (JNJ081, JNJ8114), and others have been terminated due to safety issues.
Janux's solution is elegant: attach a "mask" to the CD3-binding site that blocks T-cell engagement until the drug encounters specific enzymes found near tumors. These proteases cleave off the mask, activating the drug only where it's needed.
Early clinical data from Janux's lead program, JANX007, suggests the approach is working. In prostate cancer patients treated with the PSMA-targeting TRACTr, median radiographic progression-free survival reached 7.3 months across all evaluable subjects—comparable to the 8.6-9.3 months seen with Novartis's Pluvicto radioligand therapy—but with cytokine release syndrome largely limited to Grade 1-2 events and confined to the first treatment cycle.
Janux's Platform and Pipeline
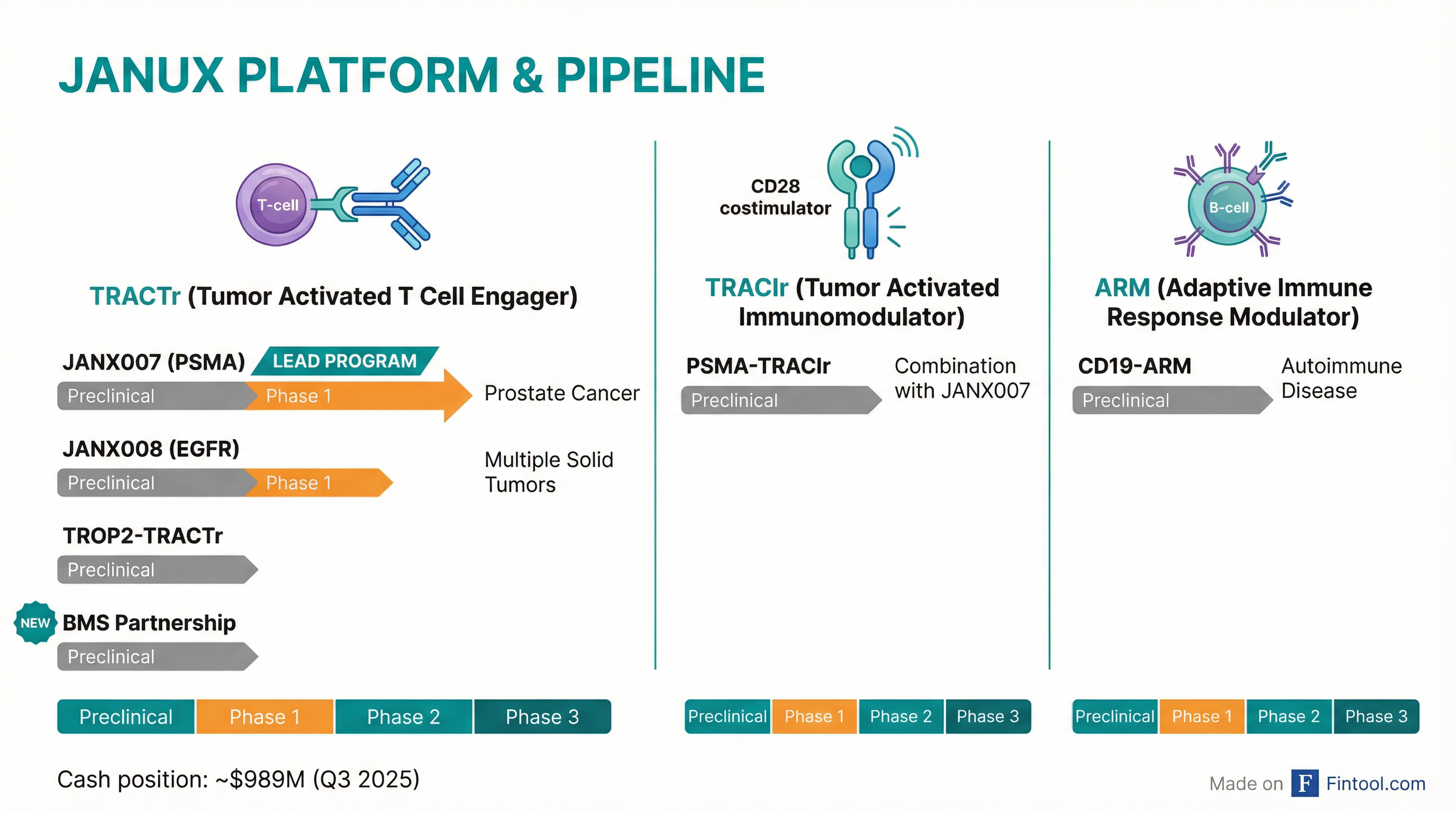
Janux has built three platforms, all leveraging its tumor-activation technology:
TRACTr (Tumor Activated T Cell Engager) — The core platform, using CD3-based T-cell engagement:
- JANX007 (PSMA): Phase 1 in metastatic castration-resistant prostate cancer. Lead program showing durable responses with manageable safety.
- JANX008 (EGFR): Phase 1 in multiple solid tumors including colorectal, head and neck, NSCLC, and pancreatic cancers.
- TROP2-TRACTr: Preclinical, targeting TROP2+ solid tumors
- BMS Partnership: New preclinical program, undisclosed target
TRACIr (Tumor Activated Immunomodulator) — CD28-based costimulation:
- PSMA-TRACIr: Preclinical, designed to combine with JANX007 for enhanced T-cell activation
ARM (Adaptive Immune Response Modulator) — Expanded into autoimmune disease:
- CD19-ARM: Preclinical for autoimmune conditions, showing rapid and durable B-cell depletion in non-human primates
This isn't Janux's first big pharma partnership. In 2020, Merck signed a deal potentially worth up to $500 million to develop T-cell engagers using Janux's technology. Merck triggered a $10 million milestone payment in mid-2025 when the first patient was dosed in the lead collaboration program.
BMS's Oncology Shopping Spree
Bristol Myers has been aggressively rebuilding its oncology pipeline as legacy products face generic competition. In 2024 alone, the company deployed over $23 billion on acquisitions:
| Acquisition | Target | Value | Strategic Rationale |
|---|---|---|---|
| Karuna | Cobenfy (schizophrenia) | $14.0B | Neuropsychiatry expansion |
| Mirati | Krazati (KRAS inhibitor) | $4.8B | Lung cancer, targeted oncology |
| RayzeBio | Actinium radiopharmaceuticals | $4.1B | Solid tumor RPT platform |
| BioNTech | BNT327 (PD-L1/VEGF bispecific) | Partnership | Next-gen IO backbone |
The Janux deal extends BMS's reach into tumor-activated bispecifics—a category the company sees as potentially transformative. BMS CEO Chris Boerner has emphasized the importance of "first or second" positioning in new therapeutic categories, citing Opdivo's experience in immuno-oncology.
In October 2025, BMS also entered a definitive agreement to acquire Orbital Therapeutics for in vivo CAR-T technology, and licensed a radiopharmaceutical from Philochem targeting prostate cancer—the same indication as Janux's lead program.
Financial Position and What's Next
Janux ended Q3 2025 with approximately $989 million in cash, cash equivalents, and short-term investments—providing substantial runway to advance its clinical programs. The BMS upfront payment adds another $50 million to the balance sheet, with potential for additional milestones as the program advances.
| Financial Metric | Q3 2025 |
|---|---|
| Cash & Investments | $989.0M |
| R&D Expenses (Quarterly) | $34.6M |
| G&A Expenses (Quarterly) | $10.6M |
| Net Loss (Quarterly) | $24.3M |
Near-term catalysts include:
- JANX007: Phase 1b taxane-naïve expansion data, dose optimization updates
- JANX008: Expansion cohort data in multiple solid tumor types
- CD19-ARM: IND-enabling studies for autoimmune indications
The company is also evaluating JANX007 in combination with darolutamide for pre-Pluvicto mCRPC patients—the largest and fastest-growing segment due to increased use of androgen receptor inhibitors earlier in the treatment pathway.
The Bottom Line
Bristol Myers is betting that Janux has cracked the code on T-cell engagers in solid tumors. If the tumor-activation technology proves out, the partnership could yield a drug that works in multiple cancer types while avoiding the toxicities that have sunk competitor programs.
For Janux, the deal provides validation of its platform and non-dilutive capital to advance its pipeline. The company now has partnerships with two of the world's largest pharmaceutical companies—Merck and Bristol Myers—both betting on the same tumor-activated approach.
JANX shares remain down more than 60% from their 52-week high of $47.58, reflecting broader biotech sector weakness and investor skepticism following mixed data updates in 2025. Today's deal may not fully rehabilitate the stock, but it provides concrete evidence that big pharma sees real value in Janux's technology.
Related Companies: Bristol Myers Squibb · Janux Therapeutics · Merck · Amgen · Novartis