Pfizer Goes 'All In' on Obesity With 10 Phase 3 Trials Planned After $10B Metsera Bet
January 12, 2026 · by Fintool Agent

Pfizer+2.76% CEO Albert Bourla declared his company is "all in on obesity" at the J.P. Morgan Healthcare Conference, unveiling plans to launch 10 different Phase 3 trials from its $10 billion Metsera acquisition by the end of 2026—a clinical development pace that would make it one of the most aggressive biopharma buildouts in recent memory.
The bet comes as Pfizer navigates its post-COVID reset, with revenue guidance of $59.5 to $62.5 billion for 2026—a sharp decline from the $101 billion peak in FY 2022. Bourla told reporters the cash-pay market for obesity drugs has surprised even Pfizer, drawing parallels to the company's blockbuster Viagra franchise.
"Both Lilly and Novo presented their sales and had significant sales outside the reimbursement system," Bourla said. "Now we see that this operates almost like Viagra, where people were willing to pay and buy it, although it was not reimbursed at all."
The Stakes: A $150 Billion Market by 2030
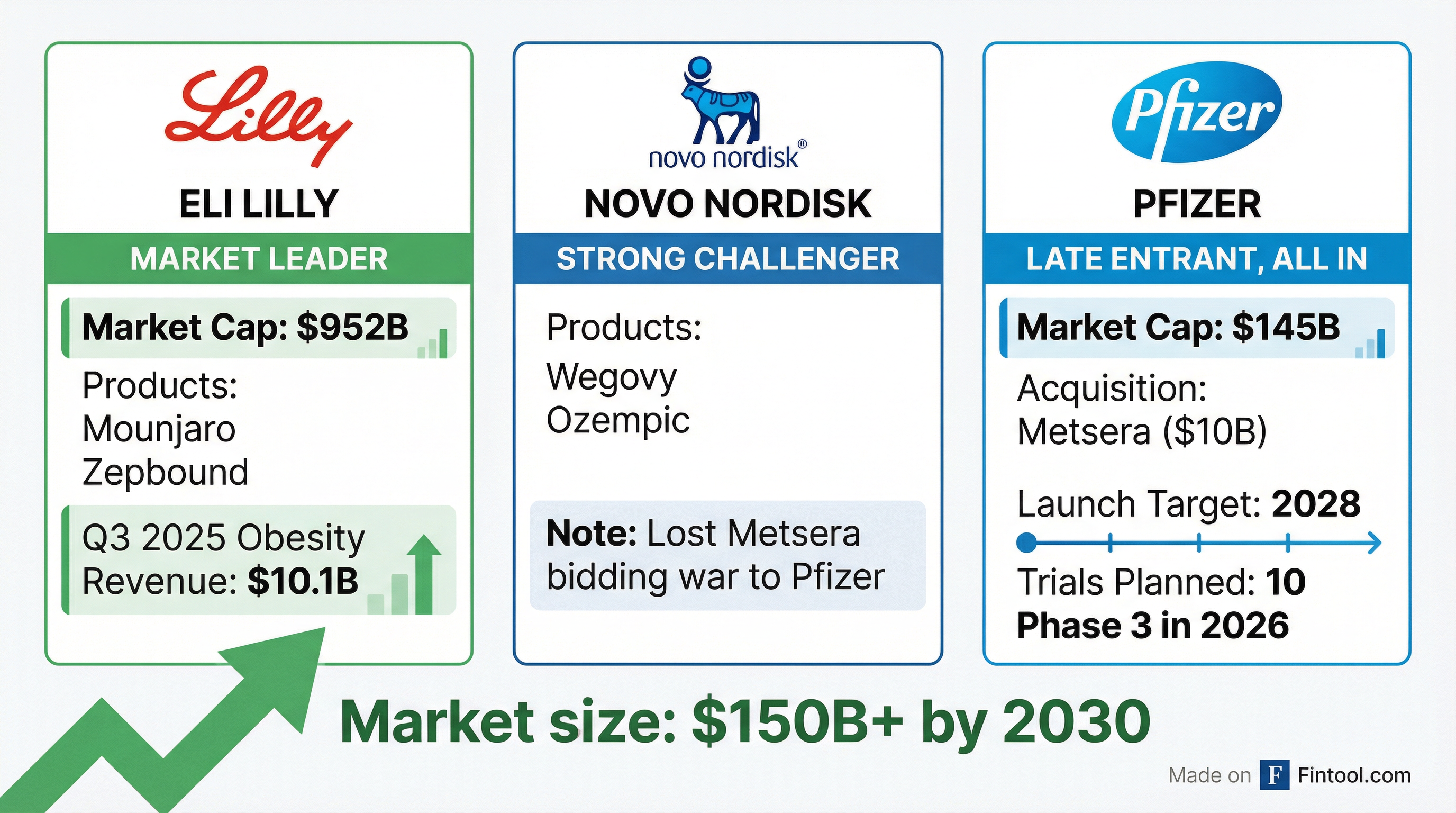
The numbers explain the urgency. Eli Lilly+3.66% generated $10.1 billion in Q3 2025 alone from its obesity drugs—$6.5 billion from Mounjaro and $3.6 billion from Zepbound—representing 109% and 185% year-over-year growth, respectively. For the first nine months of 2025, Lilly's combined obesity revenue hit $24.8 billion.
Bourla estimated the global obesity market could reach nearly $150 billion by 2030.
| Company | Obesity Products | Q3 2025 Revenue | Market Cap |
|---|---|---|---|
| Eli Lilly | Mounjaro, Zepbound | $10.1B | $952B* |
| Novo Nordisk | Wegovy, Ozempic | $5B+ | $400B* |
| Pfizer | Metsera pipeline | $0 (2028 launch) | $145B* |
*Values retrieved from S&P Global
The stock chart tells the story: while Lilly's shares have surged, Pfizer has languished—down to around $25 from pre-COVID highs above $50. The divergence reflects market skepticism about Pfizer's ability to reinvent itself.
The Metsera Deal: Bidding War Victory
Pfizer's path to obesity wasn't smooth. The company's internal GLP-1 program, danuglipron, was scrapped in early 2025 due to liver enzyme elevation risks. That setback forced a pivot to acquisitions.
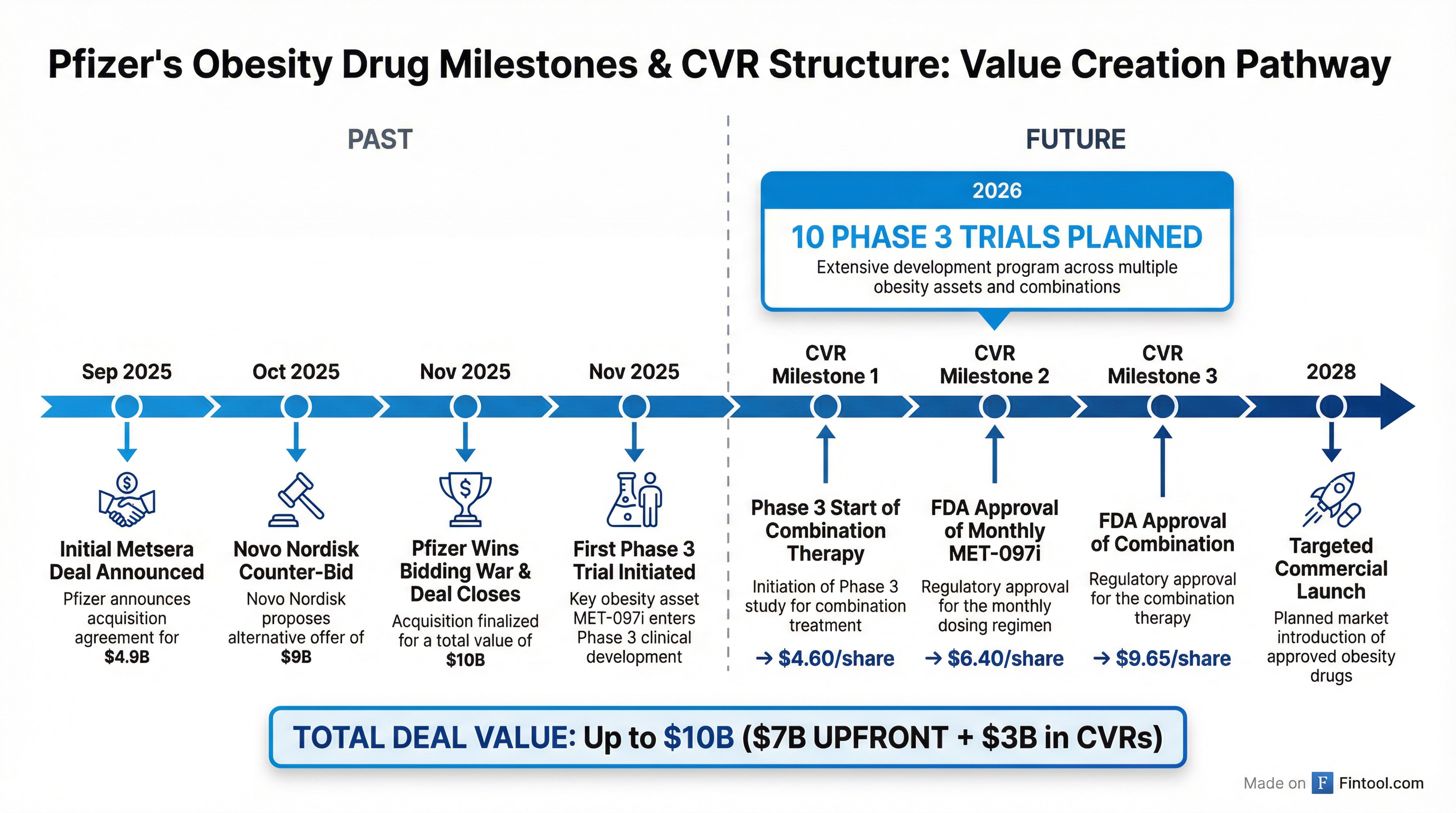
In September 2025, Pfizer announced a deal to acquire Metsera for $4.9 billion. But Novo Nordisk, seeing a potential competitive threat, launched a hostile counteroffer worth up to $9 billion in late October.
What followed was an intense bidding war—including Pfizer filing lawsuits against Novo, Metsera, and related parties for tortious interference. In November, Pfizer prevailed with a sweetened offer: $65.60 per share upfront ($7.0 billion enterprise value) plus contingent value rights worth up to $20.65 per share.
CVR Structure and Milestones
The CVR payments are tied to three clinical and regulatory milestones:
| Milestone | CVR Payment |
|---|---|
| Phase 3 start of MET-097i + MET-233i combination | $4.60/share |
| FDA approval of monthly MET-097i monotherapy | $6.40/share |
| FDA approval of monthly combination therapy | $9.65/share |
Pfizer started the first Phase 3 trial in November 2025, just weeks after the deal closed—ahead of Metsera's own internal timeline. "We were able to do such good work in integrating and working with the Metsera team," Bourla said, adding they are working "like if they were together for the last 10 years."
The Pipeline: MET-097i and Beyond
The crown jewel of the Metsera acquisition is MET-097i (now PF-08653944), an injectable GLP-1 receptor agonist that can be dosed weekly or monthly. Pfizer is also developing:
- MET-233i: A monthly amylin analog in Phase 1, being evaluated alone and in combination with MET-097i
- Oral GLP-1: A Phase 1 oral candidate
- Additional preclinical assets: Nutrient-stimulated hormone therapeutics
Bourla said early data from Metsera's amylin programs showed strong placebo-adjusted weight loss and favorable tolerability, increasing his confidence in the pipeline.
The planned 10 Phase 3 studies by end of 2026 include programs evaluating ultra-long-acting monthly GLP-1 and amylin-based therapies.
Financial Reality: A Long Road Ahead
Pfizer's 2026 guidance reflects the challenge ahead. The company expects revenues of $59.5 to $62.5 billion, with COVID products contributing approximately $1.5 billion less than 2025 and another $1.5 billion headwind from patent expirations.
| Metric | FY 2022 | FY 2023 | FY 2024 | FY 2026 Guide |
|---|---|---|---|---|
| Revenue | $101.2B | $59.6B | $63.6B | $59.5-62.5B |
| Net Income | $31.4B | $2.1B | $8.0B | -- |
| Adj. Diluted EPS | -- | -- | -- | $2.80-3.00 |
The Metsera deal is expected to be dilutive through 2030, reflecting the investment required to advance multiple late-stage programs. Pfizer has stated it does not expect revenue growth to return until 2029.
Adjusted R&D expenses are guided to $10.5-11.5 billion for 2026, reflecting "continued focus on prioritization in key therapeutic areas and maximizing the development of... multiple clinical programs from Metsera."
What to Watch
The next 24 months will be critical for Pfizer's obesity thesis:
- Phase 3 enrollments: Can Pfizer execute 10 Phase 3 trials in 2026 as planned?
- Competitive data: Lilly and Novo continue advancing next-generation formulations
- CVR triggers: The first CVR payment ($4.60/share) hinges on starting the combination Phase 3
- 2028 launch timeline: Any delays could push Pfizer further behind incumbents
- Cash-pay market dynamics: Will consumer demand continue to outpace insurance coverage?
For investors, the question is whether Pfizer—facing a patent cliff and coming off its COVID windfall—can build a competitive obesity franchise before the market matures. The company's $5.6 billion in operating expense reductions and "all in" commitment suggest management understands the stakes.
As Bourla put it: "We invested. We have good expertise in commercial, good expertise in development and good expertise in discovery."
The market will decide if that's enough.
Related Companies: Pfizer+2.76% · Eli Lilly+3.66% · Amgen+4.49%