Sanofi Pays $2.2 Billion for Dynavax in Bet on Adult Vaccines Amid Policy Uncertainty
December 25, 2025 · by Fintool Agent

Sanofi is buying Dynavax Technologies for $2.2 billion in cash, adding an approved adult hepatitis B vaccine and an early-stage shingles candidate to its portfolio as the French drugmaker expands its adult immunization business during a period of acute policy uncertainty in the U.S. vaccine market.
Dynavax shares surged 38% to $15.38 on Christmas Eve, trading within pennies of the $15.50 per share offer price—a 39% premium to the previous day's close and a 46% premium to the three-month volume-weighted average price.
The deal caps a $13.3 billion acquisition spree for Sanofi in 2025 and signals conviction that adult vaccines remain attractive despite Health Secretary Robert F. Kennedy Jr.'s campaign against childhood immunizations.
The Deal
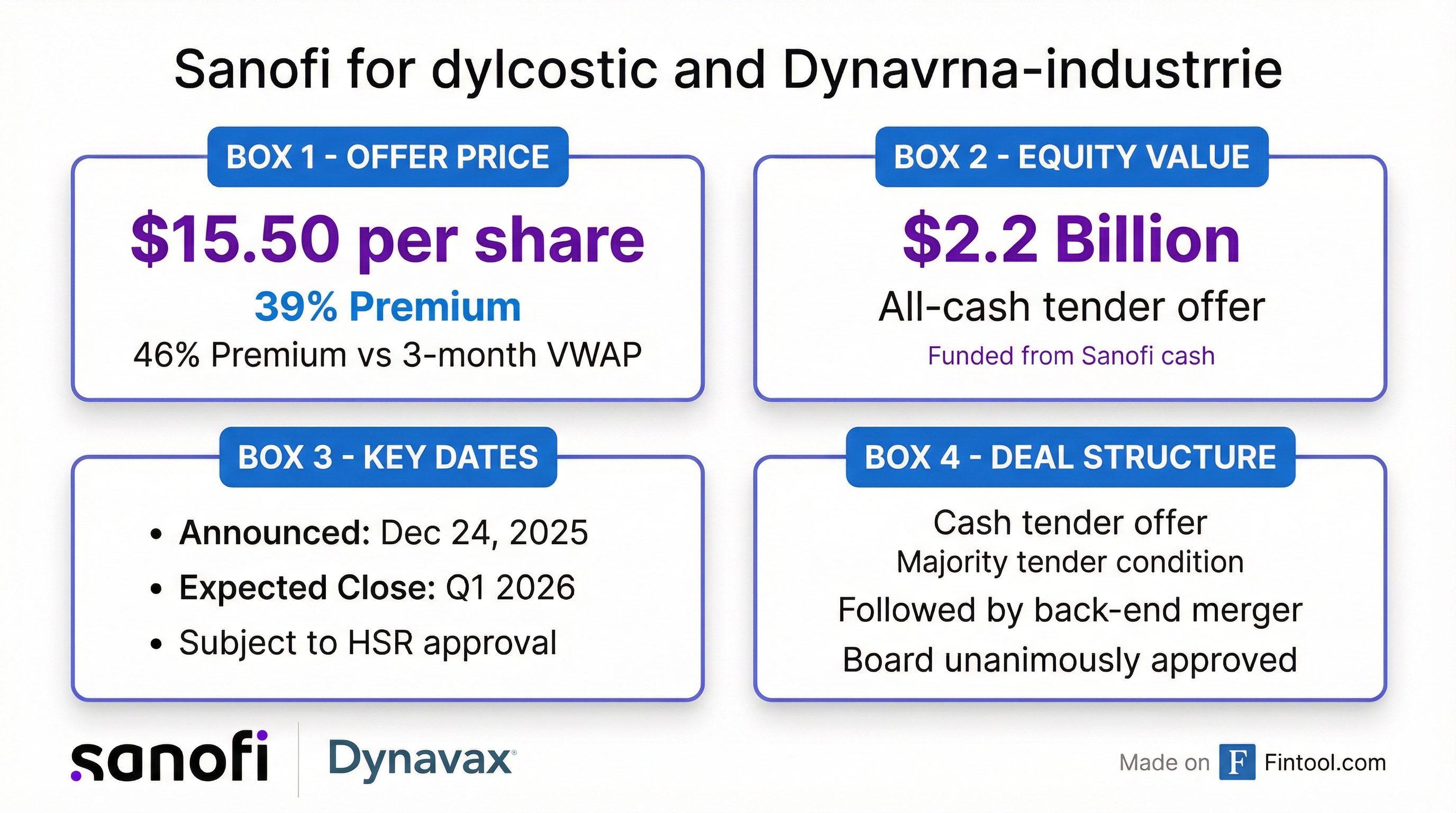
Sanofi will launch a cash tender offer at $15.50 per share, followed by a back-end merger to acquire any shares not tendered. The transaction has been unanimously approved by Dynavax's board and is expected to close in Q1 2026, subject to HSR antitrust clearance and other customary conditions.
Sanofi is funding the acquisition entirely from available cash and says the deal will not impact its 2025 financial guidance.
| Deal Metric | Value |
|---|---|
| Offer Price | $15.50/share |
| Equity Value | $2.2 billion |
| Premium to Close | 39% |
| Premium to 3-month VWAP | 46% |
| Expected Close | Q1 2026 |
| Financing | Sanofi cash |
"Joining Sanofi will provide the global scale and expertise needed to maximize the impact of our vaccine portfolio," said Ryan Spencer, Dynavax CEO.
What Sanofi Gets
The acquisition brings two primary assets: a marketed vaccine and a promising pipeline candidate.
HEPLISAV-B: A Differentiated Hepatitis B Vaccine
HEPLISAV-B is an adult hepatitis B vaccine currently marketed in the U.S., EU, and UK. It differentiates from competitors through a two-dose regimen administered over one month, versus the traditional three-dose schedule over six months.
The vaccine has been gaining share steadily, reaching 46% of the U.S. adult hepatitis B market in Q3 2025—up from 44% a year earlier—with 63% share in the retail pharmacy segment.
| HEPLISAV-B Metric | Q3 2025 | Trend |
|---|---|---|
| Net Product Revenue | $90 million | +13% YoY |
| Total U.S. Market Share | 46% | +2 ppts YoY |
| Retail Segment Share | 63% | +4 ppts YoY |
| 2025 Revenue Guidance | $315-325 million | |
| 2030 Share Target | 60%+ |
Dynavax estimates the U.S. adult hepatitis B market will grow to more than $900 million by 2030, with nearly 100 million unvaccinated American adults representing the addressable opportunity.
Z-1018: A Shingles Vaccine Challenger
Perhaps more strategically significant is Dynavax's shingles vaccine candidate, Z-1018, which is currently in Phase 1/2 clinical trials. The asset represents a potential challenger to GSK's Shingrix, which generated approximately $4 billion in annual revenue and dominates the global shingles market.
Part 1 of the Phase 1/2 study showed Z-1018 demonstrated comparable antibody and CD4+ T-cell responses to Shingrix with a favorable tolerability profile. Dynavax has now initiated Part 2 of the study in adults aged 70 and older—the highest-risk population.
"These results, which were comparable to Shingrix, support the quality and breadth of the CpG 1018 cellular immune response," said Robert Janssen, Dynavax's Chief Medical Officer.
Top-line data from Part 2 and 12-month follow-up data from Part 1 are expected in H2 2026.
Sanofi's Acquisition Spree
The Dynavax deal marks Sanofi's third major acquisition of 2025, bringing total deployed capital to approximately $13.3 billion in seven months.
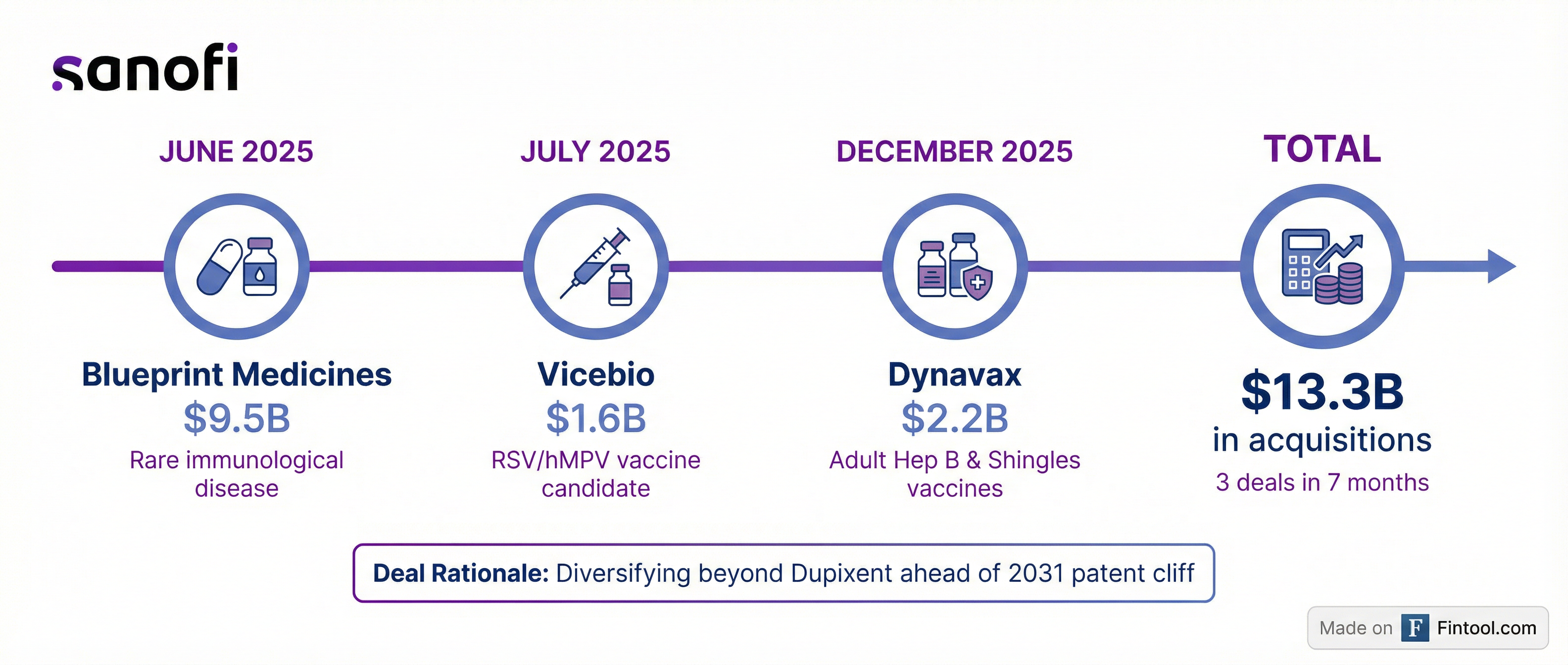
| Acquisition | Date | Value | Strategic Rationale |
|---|---|---|---|
| Blueprint Medicines | July 2025 | $9.5 billion | Rare immunological disease (systemic mastocytosis) |
| Vicebio | December 2025 | $1.6 billion | RSV/hMPV vaccine candidate (non-mRNA) |
| Dynavax | December 2025 | $2.2 billion | Adult hepatitis B and shingles vaccines |
| Total | ~$13.3 billion |
The deals reflect CEO Paul Hudson's strategy to diversify growth beyond Dupixent, Sanofi's blockbuster asthma drug that goes off patent in 2031.
"Dynavax enhances Sanofi's adult immunization presence by adding differentiated vaccines that complement Sanofi's expertise," said Thomas Triomphe, Sanofi's Executive Vice President for Vaccines.
The Policy Backdrop
The acquisition comes as vaccine makers navigate an uncertain U.S. policy environment under the Trump administration. Health Secretary Robert F. Kennedy Jr. has taken aim at childhood vaccines, dropped the universal hepatitis B vaccination recommendation for infants, and is considering further changes to immunization schedules for 2026.
The disruption has been felt across the industry:
- GSK reported a 15% year-over-year decline in U.S. Shingrix sales in Q3 2025, citing "harder-to-activate unvaccinated consumers"
- Sanofi saw its COVID-19 and flu vaccine sales decline 17% in Q3 2025
- CSL delayed plans to spin off its vaccine division, citing "heightened volatility" and declining U.S. immunization rates
However, analysts note that Sanofi's focus on adult vaccines partially insulates it from childhood immunization headwinds.
"We believe the acquisition makes sense given growing regulatory concerns around vaccines," said William Blair analyst Matt Phipps. "Sanofi is a logical acquisition partner for Dynavax given the company has extensive vaccine capabilities but the portfolio does not have an adult hepatitis B or shingles program."
Dynavax's Financial Profile
Dynavax has transformed from a development-stage company into a consistently profitable commercial operation:
| Period | Revenue | Net Income | EBITDA Margin |
|---|---|---|---|
| Q4 2023 | $51.1M | $0.2M | -9.7% |
| Q1 2024 | $47.8M | -$8.7M | -35.9% |
| Q2 2024 | $70.2M | $11.4M | 11.6% |
| Q3 2024 | $79.3M | $17.6M | 16.7% |
| Q4 2024 | $71.1M | $7.1M | 2.4% |
| Q1 2025 | $65.0M | -$96.1M | -29.7% |
| Q2 2025 | $91.9M | $18.7M | 18.8% |
| Q3 2025 | $90.0M | $26.9M | 26.1% |
Note: Q1 2025 loss driven by proxy contest expenses and Vaxart transaction costs
The company ended Q3 2025 with $648 million in cash and equivalents, down from $714 million at year-end 2024 primarily due to executing a $200 million share repurchase program.
The Pipeline Beyond Hepatitis B and Shingles
Dynavax's pipeline includes additional assets that come with the acquisition:
-
Oral COVID-19 vaccine (Vaxart license): In November 2025, Dynavax acquired an exclusive license to Vaxart's oral COVID-19 vaccine candidate, currently in a Phase 2b study with 5,400+ subjects. Dynavax has the right, but not the obligation, to proceed with development after reviewing the data expected in late 2026.
-
Plague vaccine: Fully funded by the Department of Defense, with $14 million in additional funding recently awarded. Currently in Phase 2 trials.
-
Pandemic influenza adjuvant: Phase 1/2 study completed Part 1, with Part 2 advancing optimal formulations.
What to Watch
Near-term:
- Tender offer commencement and HSR review timeline
- Q1 2026 deal closure
- Any competing bids (several law firms have already launched investigations into the transaction)
H2 2026 catalysts:
- Z-1018 shingles vaccine Part 2 data and 12-month follow-up from Part 1
- Vaxart oral COVID-19 Phase 2b data readout
Long-term:
- HEPLISAV-B market share trajectory toward 60% target
- Z-1018 path to potential Phase 3 and regulatory filing
- Sanofi's integration execution and vaccine portfolio synergies
Related Companies