Earnings summaries and quarterly performance for ARVINAS.
Executive leadership at ARVINAS.
Board of directors at ARVINAS.
Research analysts who have asked questions during ARVINAS earnings calls.
Jonathan Miller
Evercore ISI
6 questions for ARVN
Li Wang Watsek
Cantor Fitzgerald
4 questions for ARVN
Michael Schmidt
Guggenheim Securities
4 questions for ARVN
Sudan Loganathan
Stephens Inc.
4 questions for ARVN
Tazeen Ahmad
Bank of America
4 questions for ARVN
Eliana Merle
UBS
3 questions for ARVN
Ted Tenthoff
Piper Sandler & Co.
3 questions for ARVN
Terence Flynn
Morgan Stanley
3 questions for ARVN
Tyler Van Buren
TD Cowen
3 questions for ARVN
Yigal Nochomovitz
Citigroup Inc.
3 questions for ARVN
Akash Tewari
Jefferies
2 questions for ARVN
Anna
Wolfe Research
2 questions for ARVN
Blake
BTIG
2 questions for ARVN
Bradley Canino
Stifel
2 questions for ARVN
Caroline
Citi
2 questions for ARVN
Daniel Bronder
Cantor Fitzgerald
2 questions for ARVN
Derek Archila
Wells Fargo
2 questions for ARVN
Edward Tenthoff
Piper Sandler Companies
2 questions for ARVN
Francis
TD Cowen
2 questions for ARVN
Jacob
TD Cowen
2 questions for ARVN
Jeet Mukherjee
Leerink Partners
2 questions for ARVN
Kripa Devarakonda
Truist Securities
2 questions for ARVN
Luke
Goldman Sachs
2 questions for ARVN
Paul Choi
Goldman Sachs
2 questions for ARVN
Peter Lawson
Barclays PLC
2 questions for ARVN
Sarah
TD Cowen
2 questions for ARVN
Akash Bivari
Jefferies
1 question for ARVN
Amanda Acosta-Ruiz
Leerink Partners
1 question for ARVN
Andrew Berens
Leerink Partners
1 question for ARVN
Conor MacKay
BMO Capital Markets
1 question for ARVN
Daniel
Craig-Hallum
1 question for ARVN
Derek Arquilla
Wells Fargo
1 question for ARVN
Ellie Merle
UBS Group AG
1 question for ARVN
Etzer Darout
BMO Capital Markets
1 question for ARVN
Evan Seigerman
BMO Capital Markets
1 question for ARVN
Manoj
Jefferies
1 question for ARVN
Manoj Radder
Jefferies
1 question for ARVN
Matthew Biegler
Oppenheimer & Co. Inc.
1 question for ARVN
Srikripa Devarakonda
Truist Financial Corporation
1 question for ARVN
Sudan Laganathan
Stephens
1 question for ARVN
Suranjit Mukherjee
BTIG
1 question for ARVN
Recent press releases and 8-K filings for ARVN.
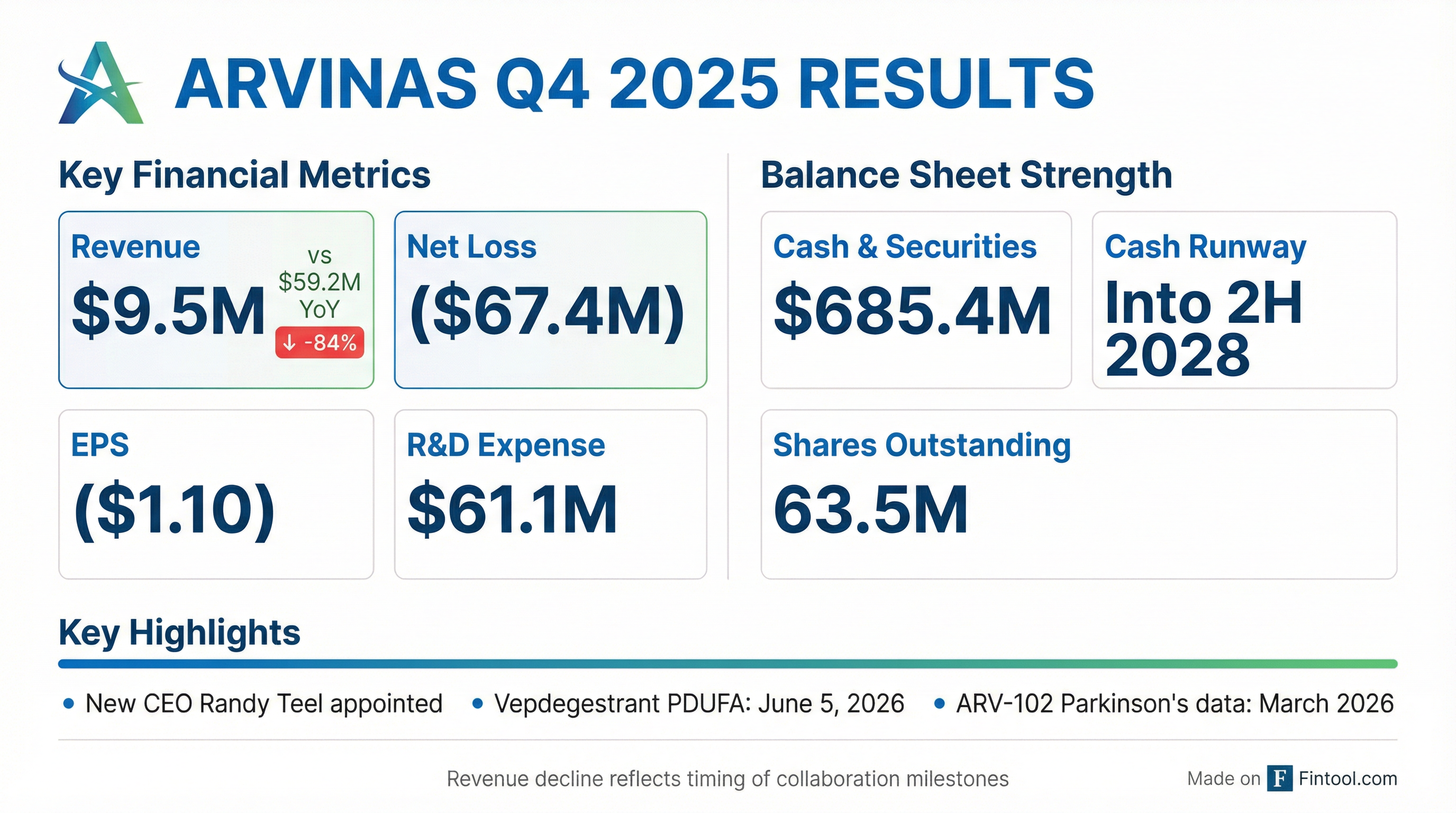
- Arvinas ended Q4 2025 with $685 million in cash, equivalents, and marketable securities , and maintains a cash runway into the second half of 2028.
- For Q4 2025, the company reported $9.5 million in revenue, a decrease from $59.2 million in Q4 2024, primarily due to a $40.3 million reduction from the Novartis license agreement.
- Arvinas has four ongoing clinical trials and recently initiated a first-in-human trial for ARV-027 , with new clinical data from Phase I trials for ARV-102, ARV-806, and ARV-393 anticipated in 2026.
- Key program milestones include the presentation of Phase 1 data for ARV-102 in Parkinson's disease patients in March, with a Phase 1b trial in PSP planned for H1 2026 , and the first data disclosure for ARV-806 expected by mid-2026.
- The company has refocused resources on its Phase I clinical programs and is working to secure a commercialization partner for vepdegestrant before its June 5th PDUFA date.
- Arvinas reported $9.5 million in revenue for Q4 2025 and $262.6 million for the full year 2025, ending the year with over $685 million in cash, equivalents, and marketable securities, and maintaining cash runway guidance into the second half of 2028.
- The company anticipates multiple data readouts and clinical advancements in 2026, including ARV-102 (LRRK2 degrader) data presentation in March and a Phase 1B trial initiation in PSP in H1 2026, ARV-806 (KRAS G12D PROTAC) data disclosure by mid-2026, and ARV-393 (BCL6 degrader) data in H2 2026.
- Arvinas is working with Pfizer to select a third party for the commercialization of vepdegestrant, aiming for an agreement before the June 5th PDUFA date.
- The company completed a $91.9 million share repurchase program in 2025, which is now suspended.
- Arvinas reported $9.5 million in revenue for Q4 2025, a decrease from $59.2 million in Q4 2024, and ended the quarter with over $685 million in cash equivalents and marketable securities, maintaining its cash runway guidance into the second half of 2028.
- The company is advancing four ongoing clinical trials and anticipates multiple data readouts and clinical advancements in 2026, including for ARV-102 (oral presentation at ADPD in March), ARV-806 (first data disclosure by mid-2026), and ARV-393 (data in H2 2026).
- Arvinas has refocused its resources on its Phase I clinical programs, which now include ARV-102, ARV-806, ARV-393, ARV-027, and ARV-6723 (entering clinic later in 2026), with a strategy to develop only highly differentiated treatments.
- The company's share repurchase program has been suspended after repurchasing approximately 10 million shares for $91.9 million as of year-end 2025.
- Arvinas reported cash, cash equivalents, and marketable securities of $685.4 million as of December 31, 2025, with a projected cash runway into the second half of 2028.
- For the full year ended December 31, 2025, the company reported a net loss of $(80.8) million and revenue of $262.6 million.
- Randy Teel, Ph.D., was appointed President, Chief Executive Officer, and Director, succeeding John Houston, Ph.D..
- The company anticipates multiple value-driving milestones in 2026, including data readouts for ARV-102, ARV-806, and ARV-393, and the initiation of a Phase 1 trial for ARV-6723.
- The Prescription Drug User Fee Act (PDUFA) action date for vepdegestrant is set for June 5, 2026, and Arvinas and Pfizer plan to identify a partner for its commercialization.
- Arvinas's partnered estrogen receptor degrader, vepdegestrant, has a PDUFA date of June 5, 2026, for ESR1 mutant metastatic breast cancer.
- The company's LRRK2 degrader, ARV-102, demonstrated 75% LRRK2 degradation and reduced neuroinflammation biomarkers in healthy volunteers. Arvinas expects to initiate a Phase 1b study in PSP in 2026 and a larger Phase 2 study by the end of 2026.
- In oncology, the BCL6 degrader ARV-393 is in a Phase 1 study with observed responders, and the KRAS G12D degrader ARV-806 showed 25 to 40 times greater preclinical potency than competitors, with clinical data anticipated in 2026.
- Arvinas reported a cash position of $788 million at the end of Q3, which is projected to fund operations well into the second half of 2028, alongside a $20 million stock repurchase in Q3.
- Arvinas's lead estrogen receptor degrader, vepdegestrant, partnered with Pfizer, has a June 5, 2026, PDUFA date for ESR1 mutant metastatic breast cancer.
- The company is advancing ARV-102, a LRRK2 degrader for Parkinson's disease and PSP, with plans to initiate a Phase 1b study in PSP in the U.S. next year and a larger Phase 2 study by the end of next year, following promising Phase 1 data showing 75% LRRK2 degradation.
- In oncology, Arvinas is progressing ARV-393 (BCL6 degrader), which has shown responders in its Phase 1 study, and ARV-806 (KRAS G12D degrader), which demonstrated 25 to 40 times greater preclinical potency than competitors.
- Arvinas reported a strong cash position of $788 million at the end of Q3, expected to fund operations well into the second half of 2028, including $20 million in stock repurchases during Q3.
- Arvinas, Inc. announced that multiple abstracts on its investigational PROTAC estrogen receptor degrader, vepdegestrant (ARV-471), have been accepted for presentation at the San Antonio Breast Cancer Symposium (SABCS) taking place from December 9–12, 2025.
- The VERITAC-2 Phase 3 study of vepdegestrant demonstrated statistically significant and clinically meaningful improvement in progression-free survival compared to fulvestrant in patients with ER+/HER2- ESR1-mutated advanced or metastatic breast cancer.
- The U.S. Food and Drug Administration (FDA) is reviewing the New Drug Application (NDA) for vepdegestrant, with a Prescription Drug User Fee Act (PDUFA) action date of June 5, 2026.
- In September 2025, Arvinas and Pfizer announced their plan to jointly select a third party for the commercialization and potential further development of vepdegestrant.
- Arvinas is undergoing a strategic "reset" (Arvinas 2.0), focusing on five early development assets, including LRRK2, KRAS G12D, and BCL6, while seeking a third-party partner for vepdegestrant's potential launch next year.
- The company reports a strong financial position with cash runway into the second half of 2028, which does not include potential future milestones or royalties from the luxdeglutamide deal with Novartis.
- The LRRK2 program is highlighted as the lead, with data from its Parkinson's disease study expected in the first few months of 2026 and an IND filing for a Progressive Supranuclear Palsy (PSP) study planned for early 2026.
- Arvinas's LRRK2 degrader has shown 75% degradation of LRRK2 in CSF and reduced neuroinflammation biomarkers in healthy volunteers, suggesting a more potent profile than competitor inhibitors.
- Arvinas is undergoing a company reset, shifting its focus to five early development assets, including LRRK2 for neurodegeneration, KRAS G12D, and BCL6 programs, with data points expected next year.
- The company is actively seeking a partner for Vepdegestrant, which had positive pivotal data and is anticipated for potential launch next year, with a decision expected before its projected June approval date.
- Arvinas maintains a strong financial position, with cash runway extending into the second half of 2028.
- The LRRK2 program is highlighted as the current lead, with a focus on Progressive Supranuclear Palsy (PSP); an IND filing for a PSP study is planned for early 2026, with potential for clinical activity measures within six months due to rapid disease progression.
- The partnership with Novartis for Luxdeglutamide includes a $1 billion upfront and milestone deal plus royalties, which is not fully factored into the current cash runway guidance.
- Arvinas is undergoing a "reset" (Arvinas 2.0), shifting its focus to five early development assets, including three currently in Phase 1 (LRRK2, KRAS G12D, BCL6) and two new programs (Kennedy's disease, HPK1) expected to enter the clinic next year.
- The company is in a strong financial position, with money into the second half of 2028. This financial runway does not contemplate potential future milestones or royalties from the $1 billion upfront and milestone deal with Novartis for Luxdeglutamide.
- The Vepdegestrant program, which showed positive pivotal data, is in the process of being transitioned to a third party for potential launch around next year if approved, with a decision date in June.
- The lead program, LRRK2, targeting Parkinson's disease (PD) and progressive supranuclear palsy (PSP), is expected to have data from its PD study in early 2026, and an Investigational New Drug (IND) application for a PSP study will be filed in early 2026.
Quarterly earnings call transcripts for ARVINAS.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more