Earnings summaries and quarterly performance for IDEAYA Biosciences.
Executive leadership at IDEAYA Biosciences.
Yujiro Hata
President and Chief Executive Officer
Andres Ruiz Briseno
Chief Accounting Officer
Darrin Beaupre
Chief Medical Officer
Joshua Bleharski
Chief Financial Officer
Michael White
Chief Scientific Officer
Stuart Dorman
Chief Commercial Officer
Board of directors at IDEAYA Biosciences.
Research analysts who have asked questions during IDEAYA Biosciences earnings calls.
Anupam Rama
JPMorgan Chase & Co.
1 question for IDYA
Ben Burnett
Stifel
1 question for IDYA
Charles Zhu
LifeSci Capital, LLC
1 question for IDYA
Joel Beatty
Robert W. Baird & Co.
1 question for IDYA
Maury Raycroft
Jefferies
1 question for IDYA
Tim Chiang
Northland Securities, Inc.
1 question for IDYA
Zegbeh Jallah
Roth Capital Partners
1 question for IDYA
Recent press releases and 8-K filings for IDYA.
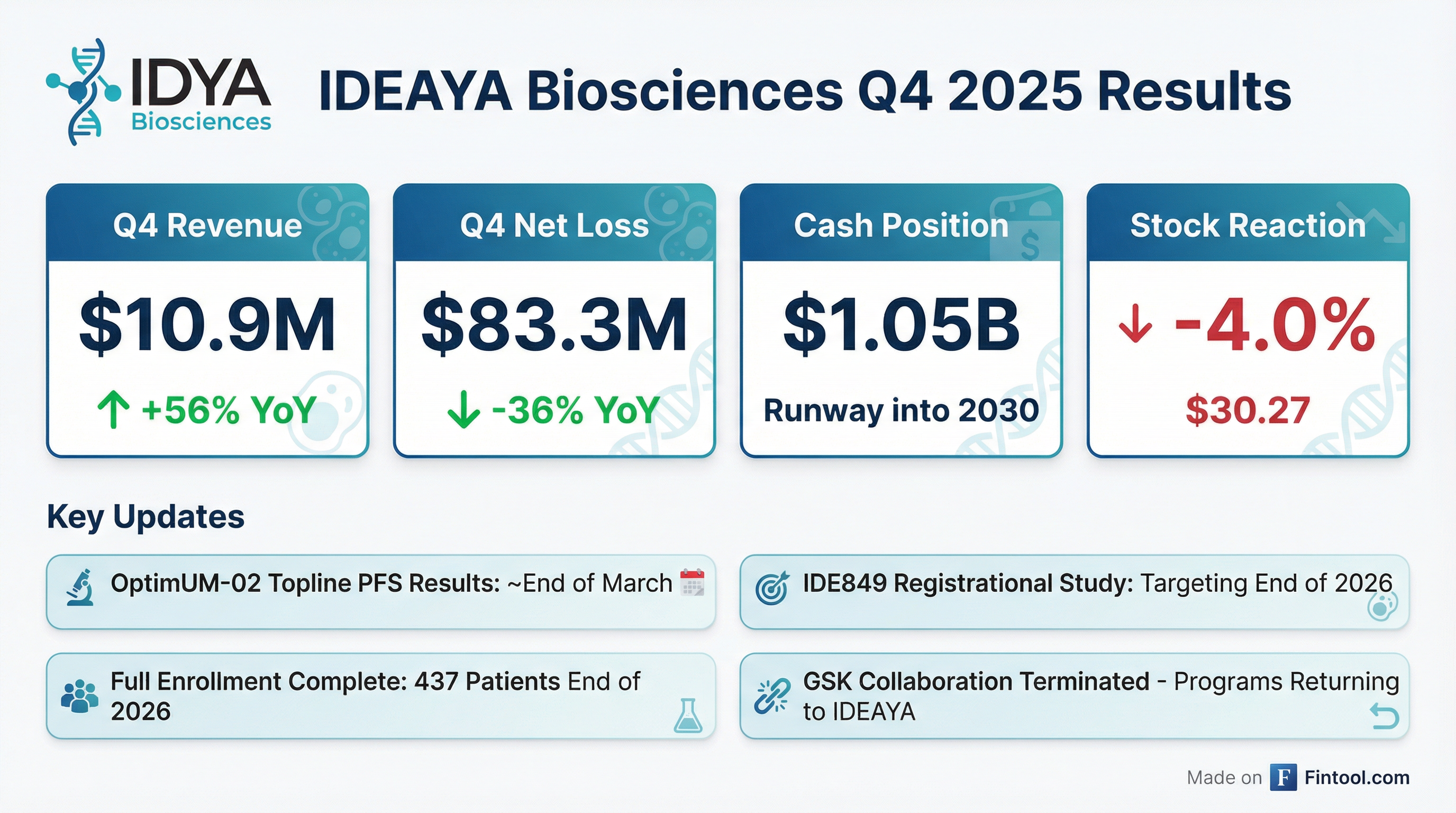
- IDEAYA Biosciences anticipates top-line results for the darovasertib Phase 3 study in first-line metastatic uveal melanoma around the very end of March. The primary endpoint for this study is median Progression-Free Survival (PFS).
- The company confirmed that the 130 required PFS events have occurred and the data cleaning process is underway, with the output expected by the end of March. The control arm PFS is expected to be 2-3 months, while the treatment arm previously showed 7 months.
- Beyond darovasertib, key pipeline updates include a clinical data update for DLL3 Topo ADC IDE-849 by the end of the year, and KAT6/7 IDE-574 has entered Phase 1 for large patient populations including breast, lung, and colorectal cancers.
- IDEAYA Biosciences expects top-line results from its Phase III randomized study of darovasertib in first-line metastatic uveal melanoma around the very end of March.
- The primary endpoint for this study is median progression-free survival (PFS), with the company anticipating a control arm PFS of 2-3 months and a treatment arm PFS of approximately 7 months.
- The 130 PFS events required for the top-line analysis have already occurred, and the company is currently in the data-cleaning process, targeting the end of March for output.
- Following the March readout, the company anticipates a timeline of approximately six months to compile data for accelerated approval filing, with the official OS interim readout expected in the first half of next year.
- Other pipeline updates include a clinical data update for DLL3 Topo ADC IDE-849 by the end of the year and KAT6/7 IDE-574 having entered Phase I.
- IDEAYA Biosciences expects top-line results for darovasertib in the first-line metastatic uveal melanoma indication on or around the very end of March. The 130 PFS events required for the interim analysis have occurred, and the company is currently in the data-cleaning process.
- The primary endpoint for this study is median progression-free survival (PFS), with the treatment arm previously demonstrating 7 months PFS compared to an expected 2-3 months for the control arm. The company also reported 21 months of overall survival (OS) from the OptimUM-01 trial.
- A clinical data update for the DLL3 Topo ADC IDE-849 program is guided for by the end of the year.
- The KAT6/7 inhibitor IDE-574 has entered Phase 1, targeting large patient populations including breast and colorectal cancer.
- IDEAYA Biosciences, a clinical-stage precision medicine oncology company, reported $1.05 billion in cash at the end of the quarter, providing a cash runway into 2030.
- Top-line data for the combination of darovasertib and crizotinib in metastatic uveal melanoma is expected at the end of March 2026, with the study having triggered the 130 events required for analysis. The success criteria for the combination arm is a 5.5-month median progression-free survival (PFS), compared to an expected 2-3 months for the control arm.
- An initial Phase I data update for the DLL3 TOPO1 ADC program is anticipated by the end of 2026, concurrently with the planned initiation of a registrational study.
- An update on the IDE397 MAT2A inhibitor in combination with Trodelvy for MTAP-deleted urothelial cancer is also expected in 2026 at a medical meeting.
- IDEAYA Biosciences, a clinical-stage precision medicine oncology company, reported $1.05 billion in cash at quarter-end, providing a financial runway into 2030.
- A significant near-term catalyst is the expected top-line data release for darovasertib in combination with crizotinib for metastatic uveal melanoma by the end of March 2026. Prior data for this combination showed a median progression-free survival of 7 months and overall survival of 21 months in first-line patients, compared to standard of care expectations of 2-3 months PFS and 13 months OS.
- Key pipeline updates include initial Phase I data for the DLL3 TOPO1 ADC and initiation of a registrational study by the end of 2026.
- An update on the IDE397 (MAT2A inhibitor) and Trodelvy combination for MTAP-deleted urothelial cancer is also anticipated in 2026.
- IDEAYA Biosciences, a clinical-stage precision medicine oncology company, anticipates a top-line data release for its lead molecule, darovasertib, in combination with crizotinib for metastatic uveal melanoma by the end of March. The success criteria for the combination arm is a 5.5-month median progression-free survival.
- The company also expects to provide an initial Phase I data update for its DLL3 TOPO1 ADC by the end of 2026 and initiate a registrational study as a monotherapy in late-line small cell or NEC by year-end.
- Updates on the IDE397 (MAT2A inhibitor) combination with Trodelvy for MTAP-deleted urothelial cancer are planned for 2026 at a medical meeting.
- IDEAYA reported $1.05 billion in cash at the end of the quarter, providing a runway into 2030.
- IDEAYA Biosciences reported approximately $1.05 billion in cash, cash equivalents, and marketable securities as of December 31, 2025, which is expected to fund operations into 2030.
- Topline progression-free survival (PFS) data from the registrational Phase 2/3 OptimUM-02 trial of darovasertib and crizotinib in first-line HLA*A2-negative metastatic uveal melanoma are expected by approximately the last week of March 2026.
- The company targets initiating a monotherapy registrational study for IDE849 (DLL3 TOP1 ADC) by the end of 2026 and received IND clearances for IDE034 and IDE574 in Q4 2025 and January 2026, respectively.
- In December 2025, GlaxoSmithKline (GSK) notified IDEAYA of its intention to terminate their Collaboration, Option and License Agreement, which will result in the transfer of the Werner Helicase (IDE275) and Pol Theta (IDE705) clinical programs back to IDEAYA.
- IDEAYA Biosciences reported approximately $1.05 billion in cash, cash equivalents, and marketable securities as of December 31, 2025, which is expected to fund operations into 2030.
- Topline results for the registrational Phase 2/3 OptimUM-02 trial of darovasertib and crizotinib combination in 1L HLA*A2-negative metastatic uveal melanoma are anticipated by approximately the last week of March.
- Darovasertib is expected to be in three randomized, Phase 3 registrational trials across all stages of uveal melanoma by H1 2026.
- The company plans to initiate a monotherapy registrational study for IDE849 (DLL3 TOP1 ADC) in the second line/refractory setting of SCLC and/or NEC by the end of 2026, with a preliminary clinical data update from its Phase 1 trial also expected by year-end 2026.
- For the fourth quarter ended December 31, 2025, IDEAYA reported a net loss of $83.3 million and collaboration revenue of $10.9 million.
- IDEAYA expects top-line results this quarter (Q1 2026) for its lead program, darovasertib, in a registrational study, aiming for potential accelerated approval filing in the U.S..
- The company plans to initiate a registrational study this year (2026) for its DLL3 TOPO-ADC program, ID849.
- IDEAYA has nine clinical stage molecules advancing, with strategic focus areas including extending the durability of TOPO-ADCs, MTAP deletion biology, and KAT6/7 inhibitors.
- IDEAYA expects top-line results this quarter (Q1 2026) from the registrational study for darovasertib in uveal melanoma, aiming for potential accelerated approval filing in the U.S.. The company also plans to initiate three randomized Phase III trials for darovasertib in H1 2026.
- The DLL3 TOPO-ADC program, IDE849, is targeted to enter a registrational study in 2026. In second-line small cell lung cancer, IDE849 showed a confirmed response rate of approximately 70% and a progression-free survival (PFS) of just over six months.
- IDEAYA is focused on MTAP deletion, a patient population found in roughly 15% of solid tumors that currently lacks approved therapies. The company is pursuing multiple strategies in this area, including combinations with MAT2A and PRMT5.
- The company's pipeline includes nine clinical stage molecules, such as the Phase I dual KAT6/7 inhibitor, IDE574, which targets tumor heterogeneity.
Fintool News
In-depth analysis and coverage of IDEAYA Biosciences.
Quarterly earnings call transcripts for IDEAYA Biosciences.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more