Earnings summaries and quarterly performance for Nurix Therapeutics.
Executive leadership at Nurix Therapeutics.
Board of directors at Nurix Therapeutics.
Research analysts covering Nurix Therapeutics.
Recent press releases and 8-K filings for NRIX.
Nurix Therapeutics Provides Update on Immunology Pipeline and Partnerships
NRIX
New Projects/Investments
Guidance Update
- Nurix is advancing a robust immunology pipeline targeting STAT6, IRAK4, and BTK, with strategic partnerships established with Sanofi and Gilead.
- The STAT6 degrader, NX-3911, partnered with Sanofi, is anticipated to complete IND-enabling studies this year (2026), potentially in the first half, with Nurix holding an option for 50/50 co-development and co-commercialization in the US. This program targets a multibillion-dollar market opportunity.
- The BTK degrader, bexobrutideg, is being expanded from oncology into autoimmune indications, including heme autoimmune, derm, and neuro applications. A new tablet formulation is currently in healthy volunteer studies, with Phase 1 data expected this year (2026).
- The IRAK4 degrader, partnered with Gilead, is completing healthy volunteer studies and has demonstrated superior tissue penetration and a safer profile compared to a competitor's program.
Feb 12, 2026, 5:00 PM
Nurix Therapeutics Highlights Immunology Pipeline Progress and Partnerships
NRIX
New Projects/Investments
- Nurix Therapeutics is advancing a robust immunology pipeline targeting BTK, IRAK4, and STAT6 with degrader technology, which offers advantages over traditional small molecule inhibitors by removing the totality of protein function, including scaffolding.
- The STAT6 degrader (NX-3911), partnered with Sanofi, is in IND-enabling studies, with completion anticipated in the first half of 2026, and Nurix retains an option for a 50/50 co-development and co-commercialization deal in the US.
- NX-3911 is positioned as a potential multibillion-dollar oral treatment for Type 2 inflammatory diseases, having demonstrated rapid and selective STAT6 degradation in preclinical models.
- The BTK degrader (bexobrutideg) is undergoing development for a tablet formulation, with Phase 1 data from healthy volunteers expected in 2026 for I&I applications. The IRAK4 degrader, partnered with Gilead, is completing the MAD portion of healthy volunteer studies, showing superior tissue penetration and safety compared to competitor compounds.
Feb 12, 2026, 5:00 PM
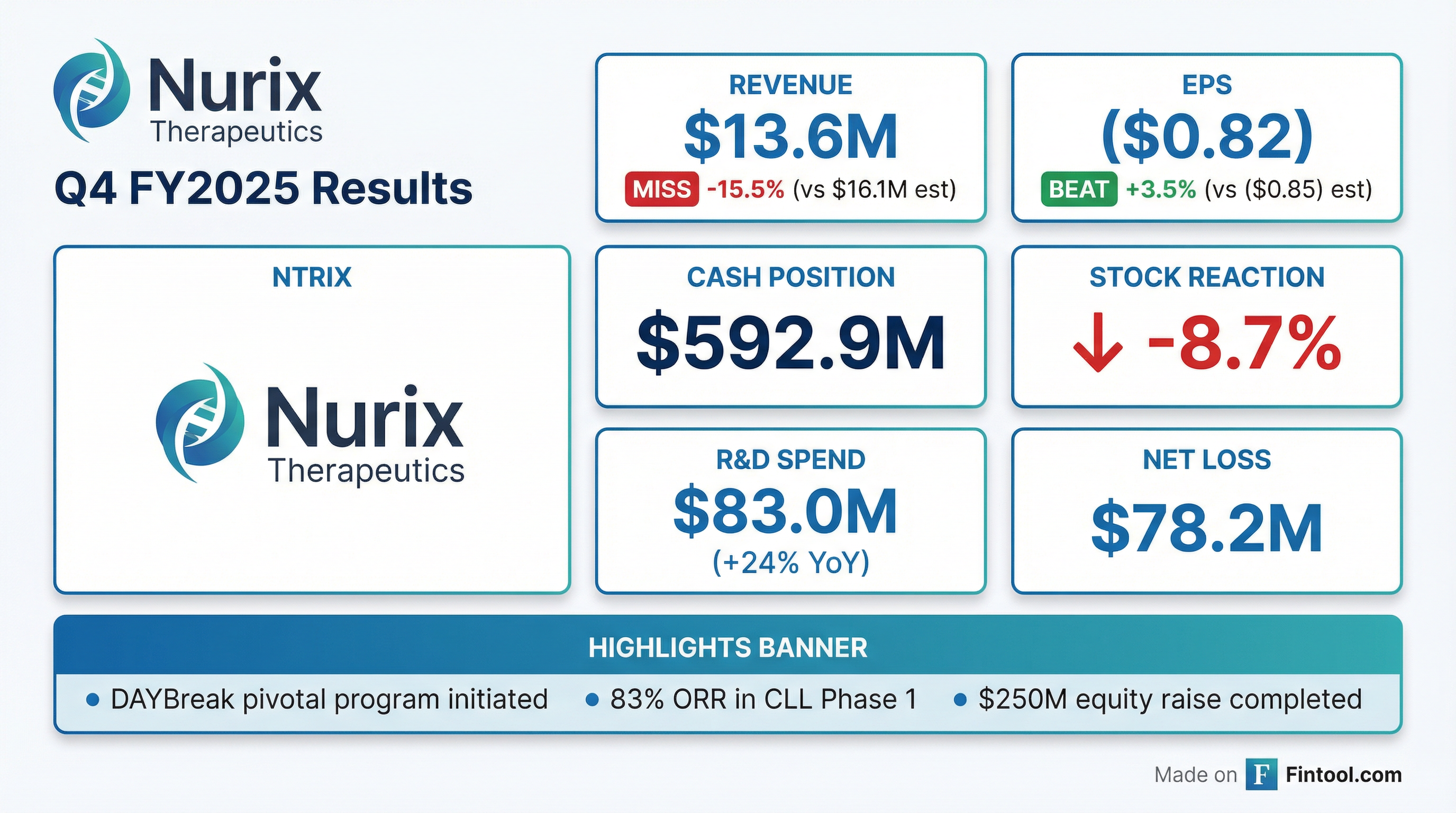
Nurix Therapeutics Reports Q4 and FY 2025 Financial Results and Provides Corporate Update
NRIX
Earnings
New Projects/Investments
Board Change
- Nurix Therapeutics reported total revenue of $13.6 million for the fourth quarter and $84.0 million for the fiscal year ended November 30, 2025, with a net loss of $78.2 million or ($0.82) per share for the quarter and $264.5 million or ($3.05) per share for the fiscal year.
- The company initiated the DAYBreak™ registrational program for bexobrutideg in relapsed/refractory CLL, supported by Phase 1 data presented at ASH 2025 showing an 83% objective overall response rate and 22.1 months median progression-free survival.
- As of November 30, 2025, Nurix held $592.9 million in cash, cash equivalents, and marketable securities, following a $250.0 million underwritten registered offering completed in October 2025.
- Nurix also provided updates on its pipeline, including differentiated preclinical data for the IRAK4 degrader GS-6791 (partnered with Gilead) and new translational data for the CBL-B inhibitor NX-1607.
Jan 28, 2026, 9:03 PM
Nurix Therapeutics Reports Fourth Quarter and Fiscal Year 2025 Financial Results
NRIX
Earnings
New Projects/Investments
Board Change
- Nurix Therapeutics reported total revenue of $84.0 million and a net loss of $264.5 million or ($3.05) per share for the fiscal year ended November 30, 2025. The company held $592.9 million in cash, cash equivalents, and marketable securities as of November 30, 2025.
- The company initiated the DAYBreak™ registrational program for bexobrutideg in relapsed/refractory CLL and presented Phase 1 results at ASH 2025, demonstrating an 83% objective overall response rate and 22.1 months progression-free survival.
- In October 2025, Nurix completed a $250.0 million underwritten registered offering of common stock and appointed Roger Dansey, M.D., to its Board of Directors in November 2025.
- During the fiscal year ended November 30, 2025, Nurix achieved research milestones of $7.0 million from Sanofi and $5.0 million from Pfizer, and a clinical milestone of $5 million from Gilead.
Jan 28, 2026, 9:01 PM
Nurix Therapeutics Presents Strong Clinical Data for Bexabrutadeg and Pipeline Updates
NRIX
New Projects/Investments
Guidance Update
- Nurix Therapeutics reported robust Phase 1A results for its lead BTK degrader, Bexabrutadeg, in heavily pre-treated CLL patients, achieving an 83% objective overall response rate and a median progression-free survival of 22.1 months.
- The company has initiated pivotal trials for Bexabrutadeg, including the Daybreak 201 study for accelerated approval and a modified Phase 3 head-to-head trial against Pirtobrutinib.
- Nurix strengthened its financial position in 2025 with a $250 million follow-on offering and ended the year with approximately $650 million in cash and investments.
- Significant pipeline progress includes ongoing Phase 1 studies for an IRAK4 degrader with Gilead and IND-enabling studies for a STAT6 degrader with Sanofi, with data and IND filings anticipated in 2026.
Jan 13, 2026, 12:30 AM
Nurix Therapeutics Presents 2025 Achievements and 2026 Outlook at JPMorgan Conference
NRIX
New Projects/Investments
Guidance Update
- Nurix Therapeutics highlighted a "terrific 2025," marked by entering pivotal trials for its lead BTK degrader, bexobrutideg, and presenting robust Phase 1A results at ASH, including an 83% objective overall response rate and 22.1 months progression-free survival in heavily pre-treated CLL patients.
- The company significantly strengthened its financial position in 2025 with a $250 million follow-on offering, concluding the year with over $650 million in cash and investments.
- Nurix modified its Phase 3 confirmatory trial for bexobrutideg to a head-to-head comparison against pirtobrutinib, aiming to demonstrate the superiority of degraders.
- For its pipeline, Nurix anticipates data from its IRAK4 degrader program with Gilead in 2026 and an IND filing for its STAT6 degrader with Sanofi also in 2026.
- The goal is to fully enroll the DAYBreak 201 pivotal trial by the end of 2026, with data expected in 2027.
Jan 13, 2026, 12:30 AM
Nurix Therapeutics Provides 2025 Highlights and 2026 Outlook at JPMorgan Healthcare Conference
NRIX
New Projects/Investments
Guidance Update
- Nurix Therapeutics highlighted a strong 2025, including entering pivotal trials for its lead BTK degrader, Bexabrutadeg, in CLL, and presenting 83% objective overall response rate and 22.1 months progression-free survival at ASH.
- The company strengthened its balance sheet with a $250 million follow-on offering and earned $47 million in partnership milestones, ending 2025 with approximately $650 million in cash and investments.
- For 2026, Nurix plans to fully enroll the Daybreak 201 Phase 2 trial by year-end, launch a Phase 3 head-to-head confirmatory study of Bexabrutadeg against Pirtobrutinib, and anticipates data from its IRAK4 degrader program with Gilead and an IND filing for its STAT6 degrader with Sanofi.
Jan 13, 2026, 12:30 AM
Nurix Therapeutics Outlines 2026 Goals and 2025 Achievements
NRIX
New Projects/Investments
Guidance Update
Management Change
- Nurix Therapeutics initiated the DAYBreak CLL-201 pivotal Phase 2 study for its BTK degrader, bexobrutideg, in relapsed/refractory chronic lymphocytic leukemia (r/r CLL) in October 2025, with plans to initiate a confirmatory Phase 3 trial in 2026.
- Clinical data for bexobrutideg, presented in December 2025, showed an 83% objective response rate and a median progression-free survival of 22.1 months in CLL patients.
- The company strengthened its financial position in October 2025 with $250.0 million in gross proceeds from a common stock offering, resulting in pro forma cash/investments of $663.8 million.
- Nurix plans to expand bexobrutideg into autoimmune and inflammatory indications with an IND submission targeted for 2026, and its partners Gilead and Sanofi are advancing IRAK4 and STAT6 degrader programs, respectively, with potential Phase 1 results for GS-6791 and an IND filing for NX-3911 anticipated in 2026.
Jan 12, 2026, 1:34 PM
Nurix Therapeutics Outlines 2026 Goals and Highlights 2025 Achievements
NRIX
New Projects/Investments
Guidance Update
Hiring
- Nurix Therapeutics advanced its BTK degrader, bexobrutideg, into pivotal Phase 2 development for relapsed/refractory chronic lymphocytic leukemia (r/r CLL) in October 2025, with plans to initiate a confirmatory Phase 3 trial in 2026. Clinical data from December 2025 showed an 83% objective response rate and 22.1 months median progression-free survival.
- The company strengthened its financial position in October 2025, securing $250.0 million in gross proceeds from a stock offering and $47.0 million from collaborations, resulting in pro forma cash/investments of $663.8 million as of August 31, 2025, plus offering proceeds.
- Nurix is expanding its pipeline, with Gilead initiating Phase 1 testing for the IRAK4 degrader (GS-6791) in April 2025, and Sanofi advancing the STAT6 degrader (NX-3911) into IND enabling studies in June 2025.
- Positive Phase 1a clinical data for the first-in-class CBL-B inhibitor NX-1607 were presented in late 2025, demonstrating immune activation and clinical activity, including a confirmed partial response in micro-satellite stable colorectal cancer.
Jan 12, 2026, 12:00 PM
Nurix Therapeutics Presents Positive Phase 1 Data for Bexobrutideg in CLL
NRIX
Product Launch
New Projects/Investments
Guidance Update
- Nurix Therapeutics announced new clinical data for bexobrutideg (NX-5948) in patients with relapsed or refractory Chronic Lymphocytic Leukemia (CLL) at the 67th American Society of Hematology (ASH) Annual Meeting & Exposition on December 6, 2025.
- The Phase 1a study demonstrated an objective response rate (ORR) of 83% (including two complete responses), a median progression-free survival (PFS) of 22.1 months, and a median duration of response (DOR) of 20.1 months.
- The 600 mg dose was selected as the recommended Phase 2 dose (RP2D) due to a higher ORR of 83.3% and suggested longer PFS in the randomized Phase 1b cohort, while maintaining a well-tolerated safety profile.
- A Phase 2 clinical trial (DAYBreak-CLL-201) is currently enrolling globally, with a confirmatory Phase 3 trial planned for initiation in H1 2026.
Dec 9, 2025, 11:05 AM
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more