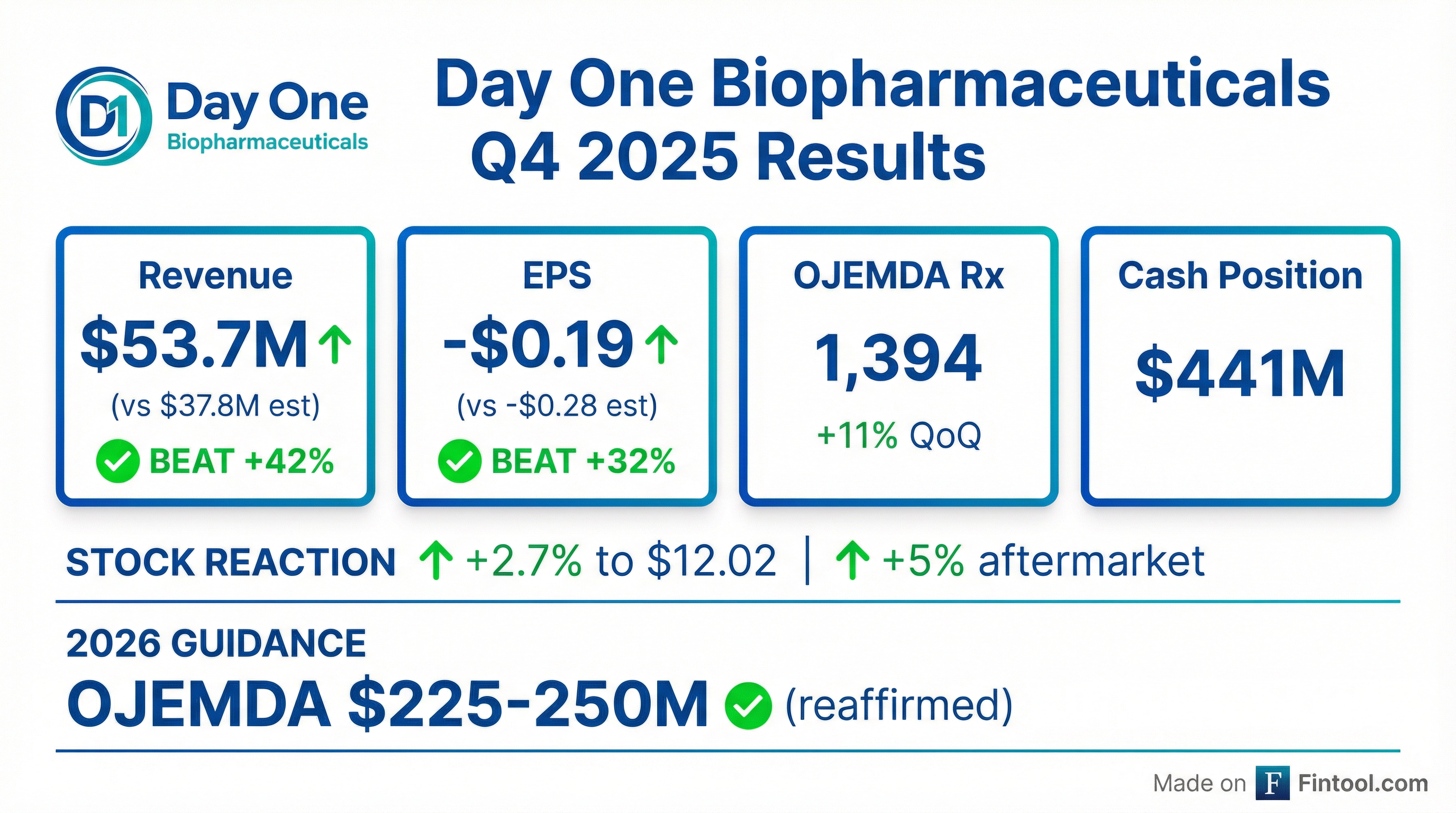
Earnings summaries and quarterly performance for Day One Biopharmaceuticals.
Executive leadership at Day One Biopharmaceuticals.
Board of directors at Day One Biopharmaceuticals.
Research analysts who have asked questions during Day One Biopharmaceuticals earnings calls.
Andres Maldonado
H.C. Wainwright & Co.
5 questions for DAWN
Alec Stranahan
Bank of America
3 questions for DAWN
Ami Fadia
Needham & Company, LLC
3 questions for DAWN
Andrea Newkirk
Goldman Sachs
3 questions for DAWN
Anupam Rama
JPMorgan Chase & Co.
3 questions for DAWN
Tara Bancroft
TD Cowen
3 questions for DAWN
Brittany Socha
Piper Sandler
2 questions for DAWN
Joseph Catanzaro
Wolfe Research, LLC
2 questions for DAWN
Nick Lorusso
TD Cowen
2 questions for DAWN
Poorna Kannan
Needham & Company
2 questions for DAWN
Priyanka Grover
JPMorgan Chase & Co.
2 questions for DAWN
Soumit Roy
JonesTrading
2 questions for DAWN
Andrea Tan
Goldman Sachs
1 question for DAWN
Recent press releases and 8-K filings for DAWN.
- BreezeBio, formerly GenEdit, announced the close of $60 million in Series B financing to advance its internal therapeutic programs and expand its NanoGalaxy delivery platform.
- The company is transitioning from a delivery platform to a therapeutics company, with its lead candidate, BRZ-101, for type 1 diabetes, advancing into IND-enabling studies.
- The Series B financing was led by new investors Yuanta Investment and DSC Investment, with participation from existing investors including DAYLI Partners and Korea Investment Partners.
- BreezeBio continues its multi-year collaboration and licensing agreement with Genentech, having achieved an initial milestone and associated payment last year.
- Day One Biopharmaceuticals reported net product revenue of $52.8 million for Q4 2025 and $155.4 million for the full year 2025, representing a 172% increase year-over-year.
- The company reiterated its 2026 OJEMDA net product revenue guidance of $225 million-$250 million.
- Day One ended 2025 with $441 million in net cash and no debt, and completed the acquisition of Mersana in January 2026, integrating the Emi-Le program into its pipeline.
- Long-term follow-up data from the FIREFLY-1 trial for OJEMDA, presented in November 2025, confirmed a 53% objective response rate and a median time to next treatment (TTNT) of 42.6 months, reinforcing its clinical impact in pediatric low-grade glioma.
- Day One Biopharmaceuticals reported $155.4 million in full-year 2025 net product revenue for OJEMDA, representing a 172% increase over 2024, with Q4 2025 revenue reaching $52.8 million.
- The company provided 2026 OJEMDA net product revenue guidance of $225 million-$250 million, implying over 50% year-over-year growth at the midpoint, and ended 2025 with approximately $441 million in net cash and no debt.
- Updated 3-year FIREFLY-1 data for OJEMDA demonstrated a 53% objective response rate and a median time to next treatment (TTNT) of 42.6 months in pediatric low-grade glioma.
- Key pipeline advancements include the acquisition of Mersana and its lead program Emi-Le for adenoid cystic carcinoma, with additional clinical data expected mid-2026, and progress on the DAY301 program with an update anticipated in the second half of 2026.
- Day One Biopharmaceuticals reported $155.4 million in net product revenue for OJEMDAT™ (tovorafenib) in 2025, marking a 172% increase.
- The company provided 2026 net product revenue guidance for OJEMDAT™ between $225 million and $250 million.
- Day One Biopharmaceuticals concluded 2025 with a strong financial position, holding $441 million in cash.
- Key pipeline milestones expected include FIREFLY-2 trial enrollment completion in 1H 2026, a Tovorafenib EMA regulatory decision in 2026, and Phase 1 data for Emi-Le in mid-2026.
- Day One Biopharmaceuticals reported Q4 2025 net product revenue of $52.8 million and full-year 2025 net product revenue of $155.4 million, marking a 172% increase over 2024.
- The company provided 2026 OJEMDA net product revenue guidance of $225 million-$250 million, with the midpoint implying over 50% year-over-year growth.
- Day One ended 2025 with approximately $441 million in net cash and no debt, and completed the acquisition of Mersana in January 2026.
- Key pipeline advancements include the expected completion of FIREFLY-2 trial enrollment in the first half of 2026 with a mid-2027 top-line readout, and additional clinical data for Emi-Le in mid-2026.
- Day One Biopharmaceuticals reported OJEMDA net product revenues of $52.8 million for the fourth quarter of 2025 and $155.4 million for the full year 2025, representing 172% year-over-year growth.
- The company reaffirmed its 2026 U.S. OJEMDA net product revenue guidance of $225 million to $250 million, which indicates over 50% growth at the midpoint compared to 2025.
- The net loss for Q4 2025 was $21.3 million, and for the full year 2025, it was $107.3 million.
- As of December 31, 2025, the company's cash, cash equivalents, and short-term investments totaled $441.1 million.
- Key pipeline advancements include anticipated full enrollment in the pivotal Phase 3 FIREFLY-2 trial in the first half of 2026, updated Phase 1 clinical data for Emi-Le expected mid-2026, and initial clinical data for DAY301 planned for the second half of 2026.
- Day One Biopharmaceuticals reported preliminary unaudited full year 2025 net product revenues of $155.4 million, marking 172% year-over-year growth, and Q4 2025 net product revenue of $52.8 million, a 37% sequential increase over Q3.
- The company ended 2025 in a strong financial position with more than $440 million in cash and no debt.
- For 2026, Day One is guiding to OJEMDA net product revenue of $225 million-$250 million.
- Day One completed the acquisition of Mersana last week, adding the Emmy Lee program, which is projected to generate $300 million or more in revenue and has a potential three-year path to approval.
- Enrollment in the FIREFLY-2 trial for frontline pLGG is expected to be complete in the first half of 2026, with data anticipated in mid-2027 and potential approval in 2028.
- Day One Biopharmaceuticals reported preliminary unaudited Q4 2025 net product revenue for Ojenda of $52.8 million, contributing to a full-year 2025 net product revenue of $155.4 million, representing a 172% year-over-year increase. For 2026, the company is guiding for Ojenda net product revenue of $225-$250 million.
- The company completed the acquisition of Mersana in Q4 2025, adding new pipeline programs including Emmy Lee and Day 301. Emmy Lee, targeting adenoid cystic carcinoma (ACC), has the potential to generate $300 million or more in revenue, with expanded Phase 1 data expected in mid-2026. Early clinical updates for Day 301 are anticipated in H2 2026.
- Day One ended 2025 with over $440 million in cash and no debt. The company projects a clear path to achieve more than $1 billion in revenue based on the potential sales from Ojenda and Emmy Lee alone.
- Enrollment in the Firefly-2 trial for frontline pediatric lobar glioma (PLGG) is expected to be completed in the first half of 2026, with potential approval anticipated in 2028. Additionally, an EME approval decision for Ojenda outside the U.S. is expected in 2026 through its partner Ipsen.
- Day One Biopharmaceuticals reported unaudited full-year 2025 net product revenues of $155.4 million, with Q4 net product revenue reaching $52.8 million, and ended 2025 with more than $440 million in cash and no debt.
- The company provided 2026 guidance for Ojenda net product revenue of $225-$250 million.
- Day One recently completed the acquisition of Mersana, adding the Emmy Lee program to its portfolio, which has the potential to generate $300 million or more in revenue. Expanded Phase 1 data for Emmy Lee is expected in mid-2026.
- Enrollment for the Firefly-2 trial, expanding Ojenda into frontline pediatric lobar glioma (PLGG), is anticipated to be complete in the first half of 2026, with data expected in mid-2027 and potential approval in 2028.
- The company aims to achieve more than $1 billion in revenue based on the potential sales from Ojenda and Emmy Lee alone.
- Day One Biopharmaceuticals reported preliminary unaudited OJEMDA net product revenue of $52.8 million for Q4 2025 and $155.4 million for the full year 2025, representing 172% year-over-year growth compared to 2024.
- The company projects 2026 OJEMDA U.S. net product revenue to be between $225 million and $250 million, which signifies 53% year-over-year growth at the midpoint.
- As of December 31, 2025, Day One had approximately $441.1 million in cash, cash equivalents, and short-term investments (unaudited).
- Key corporate priorities for 2026 include completing enrollment in the pivotal Phase 3 FIREFLY-2 clinical trial in the first half of 2026, delivering Phase 1 clinical data for Emi-Le by mid-2026, and providing initial data from the Phase 1a clinical trial for DAY301 in the second half of 2026.
Quarterly earnings call transcripts for Day One Biopharmaceuticals.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more