Earnings summaries and quarterly performance for Jazz Pharmaceuticals.
Executive leadership at Jazz Pharmaceuticals.
Renee Gala
President and Chief Executive Officer
Liz Henderson
Senior Vice President, Technical Operations
Neena Patil
Executive Vice President and Chief Legal Officer
Patricia Carr
Senior Vice President, Chief Accounting Officer
Philip Johnson
Executive Vice President and Chief Financial Officer
Robert Iannone
Executive Vice President, Global Head of Research & Development and Chief Medical Officer
Samantha Pearce
Executive Vice President, Chief Commercial Officer
Board of directors at Jazz Pharmaceuticals.
Anne O’Riordan
Director
Bruce Cozadd
Chair of the Board
Heather Ann McSharry
Director
Jennifer Cook
Director
Kenneth O’Keefe
Director
Laura Hamill
Director
Mark Smith
Director
Norbert Riedel
Director
Patrick Enright
Director
Patrick Kennedy
Director
Rick Winningham
Lead Independent Director
Seamus Mulligan
Director
Ted Love
Director
Research analysts who have asked questions during Jazz Pharmaceuticals earnings calls.
David Amsellem
Piper Sandler Companies
8 questions for JAZZ
Jason Gerberry
Bank of America Merrill Lynch
7 questions for JAZZ
Marc Goodman
Leerink Partners
7 questions for JAZZ
Mohit Bansal
Wells Fargo & Company
7 questions for JAZZ
Ami Fadia
Needham & Company, LLC
6 questions for JAZZ
Gary Nachman
Raymond James
6 questions for JAZZ
Joseph Thome
TD Cowen
6 questions for JAZZ
Andrea Newkirk
Goldman Sachs
5 questions for JAZZ
Annabel Samimy
Stifel Financial Corp.
5 questions for JAZZ
Gregory Renza
RBC Capital Markets
5 questions for JAZZ
Jessica Fye
JPMorgan Chase & Co.
5 questions for JAZZ
Joon Lee
Truist Securities
5 questions for JAZZ
Akash Tewari
Jefferies
3 questions for JAZZ
Amy Li
Jefferies Financial Group Inc.
3 questions for JAZZ
Michael Riad
Morgan Stanley
3 questions for JAZZ
Anastasia
Jefferies
2 questions for JAZZ
Brian Skorney
Robert W. Baird & Co.
2 questions for JAZZ
David Hong
Deutsche Bank
2 questions for JAZZ
Jeff Hung
Morgan Stanley
2 questions for JAZZ
Leonid Timashev
RBC Capital Markets
2 questions for JAZZ
Troy Langford
TD Cowen
2 questions for JAZZ
Ashwani Verma
UBS Group AG
1 question for JAZZ
Charles Duncan
Cantor Fitzgerald & Co.
1 question for JAZZ
David Hoang
Citigroup
1 question for JAZZ
Joel Beatty
Baird
1 question for JAZZ
Sean Laaman
Morgan Stanley & Co.
1 question for JAZZ
Recent press releases and 8-K filings for JAZZ.
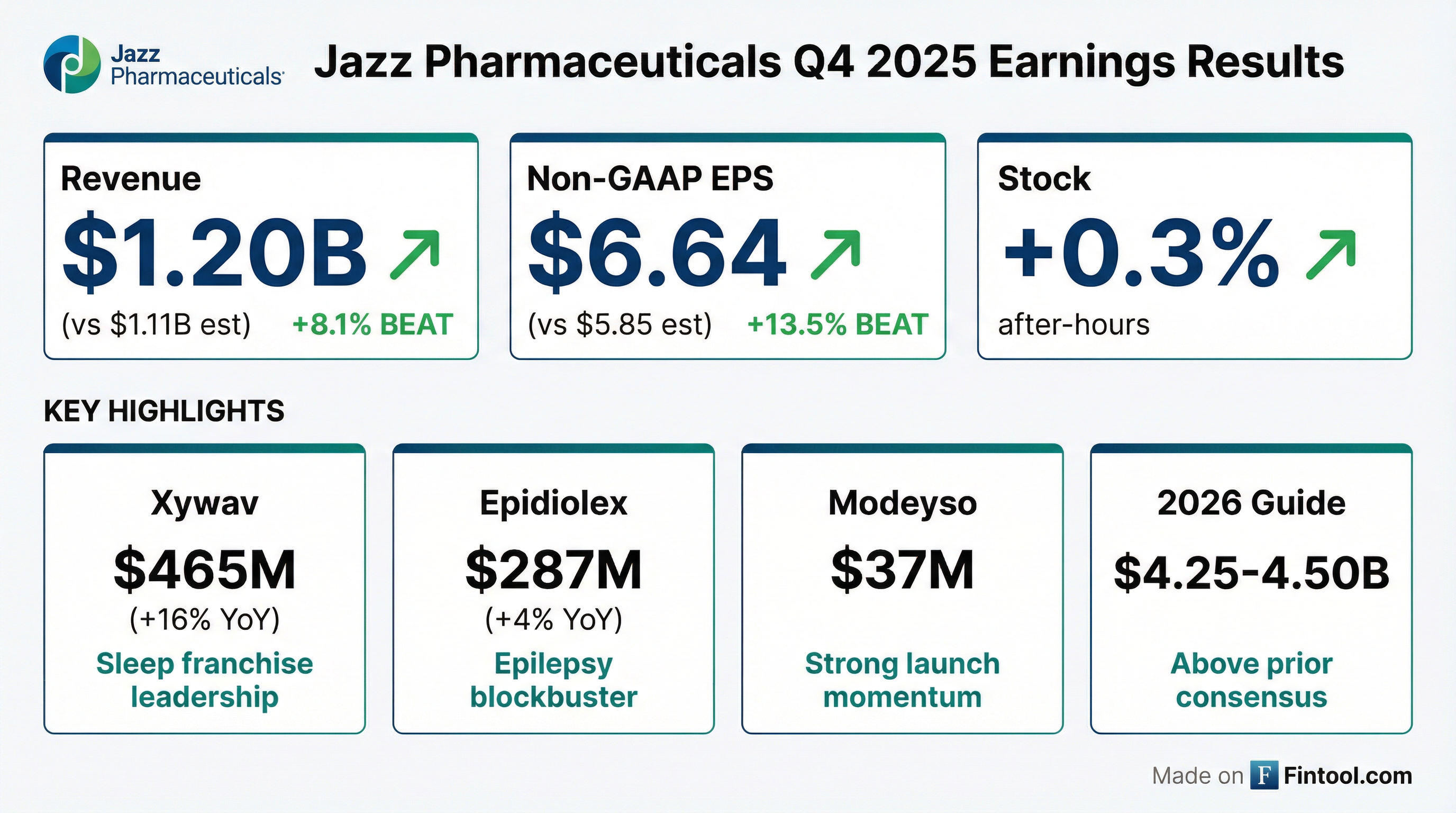
- Jazz Pharmaceuticals reported record total revenue of $4.3 billion for the full year 2025 and $1.2 billion for Q4 2025, representing 10% year-over-year growth. The company projects 2026 total revenue to be between $4.25 billion and $4.50 billion.
- Key product drivers in 2025 included XYWAV revenue of $1.7 billion (up 12%) and Epidiolex revenue of $1.1 billion (up 9%), which achieved blockbuster status. Modeyso, launched in August 2025, generated $48 million in revenue.
- Significant pipeline advancements include practice-changing data for zanidatamab in HER2-positive metastatic GEA, with a potential launch in this indication in the second half of 2026 following Breakthrough Designation.
- The company settled outstanding ANDA litigation for Epidiolex, extending its market runway into the very late 2030s, and sold a priority review voucher for $200 million in gross proceeds.
- Jazz Pharmaceuticals reported record total revenues of $1.2 billion for Q4 2025 and $4.3 billion for the full year 2025, representing 10% and 5% growth over 2024, respectively.
- XYWAV revenue grew 12% to approximately $1.7 billion for the year, and Epidiolex achieved $1.1 billion in revenue, up 9% year-over-year in 2025. Modeyso, launched in August 2025, generated $48 million in revenue by year-end.
- For 2026, the company expects total revenue guidance of $4.25 billion-$4.50 billion, with double-digit growth anticipated in rare oncology and epilepsy revenues, driven by Epidiolex, Modeyso, and Ziihera.
- The rare sleep franchise is projected to decline to $1.8 billion-$1.9 billion in 2026 from $2.01 billion in 2025, primarily due to generic high-sodium oxybate products and a modest step down in the Hikma royalty rate.
- Key pipeline updates include the potential launch of zanidatamab in first-line metastatic GEA in the second half of 2026, following Breakthrough Designation from the FDA, while the development of JZP441 (orexin program) has been stopped.
- Jazz Pharmaceuticals reported record total revenue of $4.3 billion for the full year 2025, with Q4 2025 revenue at $1.2 billion, marking 10% year-over-year growth and the highest revenue quarter ever. Non-GAAP adjusted EPS for 2025 was $8.38.
- For 2026, the company projects total revenue between $4.25 billion and $4.50 billion, a 2.5% growth at the midpoint compared to 2025. This outlook anticipates double-digit growth in rare oncology and epilepsy revenues, offset by a modest decrease in rare sleep revenue due to increased generic competition.
- Product highlights include Epidiolex achieving blockbuster status with $1.1 billion in revenue and XYWAV revenue growing 12% to $1.7 billion in 2025. The recently launched Modeyso generated $48 million in 2025 revenue and is projected to have a peak sales opportunity of greater than $500 million in the U.S..
- The company is advancing its pipeline, with zanidatamab receiving FDA Breakthrough Designation in GEA and a potential launch in the second half of 2026. Jazz has also sharpened its strategic focus on rare disease and significantly deleveraged its balance sheet to approximately 1.5 turns of EBITDA by the end of 2025, enabling future M&A.
- Jazz Pharmaceuticals plc reported record total revenues of $4.3 billion for the full year 2025, a 5% year-over-year increase, and $1.2 billion for Q4 2025, a 10% year-over-year increase.
- The company provided 2026 financial guidance, projecting total revenues between $4.25 billion and $4.50 billion.
- Key product performance in 2025 included Xywav revenue of $1.7 billion (+12% YoY) and Epidiolex revenue of $1.1 billion (+9% YoY).
- Modeyso generated $48 million in sales in 2025 following its August 2025 launch, with $37 million in Q4 2025.
- As of December 31, 2025, the company held $2.4 billion in cash, cash equivalents, and investments and had $5.4 billion in outstanding long-term debt.
- Jazz Pharmaceuticals reported record total revenues of $4.3 billion for the full year 2025, a 5% year-over-year increase, and $1.2 billion for the fourth quarter of 2025, up 10% year-over-year.
- In 2025, Xywav revenue reached $1.7 billion (+12% YoY) and Epidiolex revenue was $1.1 billion (+9% YoY).
- The company issued 2026 total revenue guidance between $4.25 billion and $4.50 billion, anticipating double-digit growth across its epilepsy and oncology franchises.
- Jazz Pharmaceuticals generated $1.4 billion in cash from operations in 2025 and held $2.4 billion in cash, cash equivalents, and investments as of December 31, 2025.
- Jazz Pharmaceuticals reported record fourth-quarter revenue of $1.2 billion, a 10% year-over-year increase, and adjusted EPS of $6.64, surpassing analyst estimates.
- The company provided 2026 revenue guidance in the range of $4.25 billion to $4.50 billion.
- Management plans to continue growth into 2026 through potential launches and label expansions, such as zanidatamab for GEA, and by bolstering key products like Epidiolex and Xywav.
- Despite these positive results, the company faces challenges including declining margins and recent insider selling.
- Jazz Pharmaceuticals' new CEO, Renée Galá, presented the company's refined strategy to focus on rare disease, leveraging existing expertise in rare sleep, epilepsy, and oncology, and expanding into new therapeutic areas.
- The company highlighted significant 2025 accomplishments, including Epidiolex achieving $1 billion in sales, record total revenue, successful launches of Medaso and Zepzelca in new indications, and the resolution of major litigation.
- Ziihera (zanidatamab) is positioned as a cornerstone for future growth, with positive Phase III data in GEA, a planned sBLA filing in the first half of 2026, and eligibility for Real-Time Oncology Review (RTOR).
- Jazz reported a strong financial position, generating nearly $1 billion in cash in the first nine months of 2025 and ending Q3 2025 with over $2 billion in cash and investments, supporting internal growth and corporate development.
- Looking ahead to 2026, the company expects to provide guidance in February, anticipating robust growth in non-oxybate revenue despite the entry of generic high-sodium oxybate products, and plans for one or more corporate development deals.
- Jazz Pharmaceuticals achieved over $4 billion in total revenues for the year ended December 31, 2025, meeting its guidance, and reported three $1B+ franchises including Epidiolex and Xywav each exceeding $1 billion in sales.
- Modeyso (dordaviprone) received rapid FDA approval and generated nearly $50 million in revenue for 2025, with a projected $500M+ peak sales potential.
- The company plans to submit a supplemental Biologics License Application (sBLA) for zanidatamab in 1L GEA in 1H 2026, with potential approval and launch in late 2026, representing a $2B+ opportunity.
- Jazz Pharmaceuticals also reduced leverage with a $750 million debt paydown and increased equity value by $2.8 billion (YE2025 vs. YE2024).
- Jazz Pharmaceuticals achieved record total revenue in 2025, with Epidiolex reaching $1 billion in sales for the first time, and successful launches of Medaso and Zepzelca.
- The company is sharpening its strategic focus on rare disease, leveraging existing capabilities in rare sleep, rare epilepsy, and rare oncology, and expanding into new therapeutic areas.
- Jazz plans to submit its sBLA filing for zanidatamab (Ziihera) in gastroesophageal adenocarcinoma (GEA) in the first half of 2026, with potential for approval and launch before year-end, projecting it as a $2 billion-plus commercial opportunity.
- The company reported a strong financial position, generating nearly $1 billion in cash in the first nine months of 2025 and ending Q3 2025 with over $2 billion in cash and investments.
- Jazz anticipates robust growth in non-oxybate revenue for 2026, despite the launch of Amneal's Xyrem generic, confident in the differentiation of Xywav.
- CEO Renée Galá highlighted 2025 accomplishments, including Epidiolex achieving $1 billion in sales and the company reaching record total revenue.
- Jazz Pharmaceuticals is sharpening its strategic focus on rare diseases, aiming to build robust billion-dollar franchises in existing areas like rare sleep, epilepsy, and oncology, as well as new rare therapeutic areas.
- The company's lead oncology asset, zanidatamab (Ziihera), is a potential $2 billion-plus commercial opportunity with an sBLA filing planned for H1 2026 and potential approval and launch later in the year for HER2-positive first-line GEA.
- Jazz reported a strong financial position, generating nearly $1 billion in cash in the first nine months of 2025 and ending Q3 2025 with over $2 billion of cash and investments.
- While Amneal launched a generic high-sodium oxybate (Xyrem), Jazz's low-sodium oxybate, Xywav, is strongly positioned with over 16,000 patients and excellent payer contracts as of early 2026.
Quarterly earnings call transcripts for Jazz Pharmaceuticals.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more