Earnings summaries and quarterly performance for Vir Biotechnology.
Executive leadership at Vir Biotechnology.
Board of directors at Vir Biotechnology.
Research analysts who have asked questions during Vir Biotechnology earnings calls.
Patrick Trucchio
H.C. Wainwright & Co.
6 questions for VIR
Philip Nadeau
TD Cowen
6 questions for VIR
Joseph Stringer
Needham & Company
5 questions for VIR
Alec Stranahan
Bank of America
3 questions for VIR
Huidong Wang
Barclays
3 questions for VIR
Kyuwon Choi
Goldman Sachs
3 questions for VIR
Michael Ulz
Morgan Stanley
3 questions for VIR
Paul Choi
Goldman Sachs
3 questions for VIR
Sean McCutcheon
Raymond James
3 questions for VIR
Avraham Novick
Morgan Stanley
2 questions for VIR
Gena Wang
Barclays
2 questions for VIR
Josh Schimmer
Cantor Fitzgerald
2 questions for VIR
Nikola Gasic
Leerink Partners
2 questions for VIR
Roanna Ruiz
Leerink
2 questions for VIR
Alex Tranehan
Bank of America
1 question for VIR
Ali Mohammed
Leerink Partners
1 question for VIR
Avi Nova
Morgan Stanley
1 question for VIR
Billy
JPMorgan Chase & Co.
1 question for VIR
Eric Joseph
JPMorgan Chase & Co.
1 question for VIR
Hang Hu
Barclays
1 question for VIR
Rona Ruiz
Leerink Partners
1 question for VIR
Ronny Dvir
JPMorgan Chase & Co.
1 question for VIR
Recent press releases and 8-K filings for VIR.
- Vir Biotechnology, Inc. entered into an underwriting agreement on February 25, 2026, for a public offering of 17,647,059 shares of its common stock at $8.50 per share.
- The company expects to receive net proceeds of approximately $141.1 million from the offering, potentially increasing to $162.3 million if the underwriters fully exercise their option to purchase an additional 2,647,058 shares.
- The offering is anticipated to close on February 27, 2026.
- Vir Biotechnology announced the pricing of its underwritten public offering of 17,647,058 shares of common stock at $8.50 per share.
- The offering is expected to generate $150 million in gross proceeds for the company.
- The underwriters have been granted a 30-day option to purchase up to an additional 2,647,058 shares of common stock.
- The closing of the offering is expected to occur on February 27, 2026.
- Vir Biotechnology announced an underwritten public offering of $200 million of common stock, with a 30-day option for an additional $30 million, to support ongoing development.
- The company reported a recent quarterly earnings beat with revenue well above estimates, but continues to show significant net losses, negative return on equity, and rapid cash burn.
- CFO Jason O’Byrne disclosed a sale of 2,089 shares at an average price of $7.45, resulting in a 1.26% decrease in his direct ownership.
- Analyst sentiment is mixed, but includes bullish views, with Evercore reaffirming an "outperform" rating and an $18 target.
- Vir Biotechnology, Inc. announced its intention to offer $200,000,000 of shares of its common stock in an underwritten public offering.
- The company also intends to grant the underwriters a 30-day option to purchase up to an additional $30,000,000 of shares of its common stock.
- All shares in the proposed offering will be offered by Vir Biotechnology.
- The proposed offering is subject to market and other conditions, and there is no assurance regarding its completion, actual size, or terms.
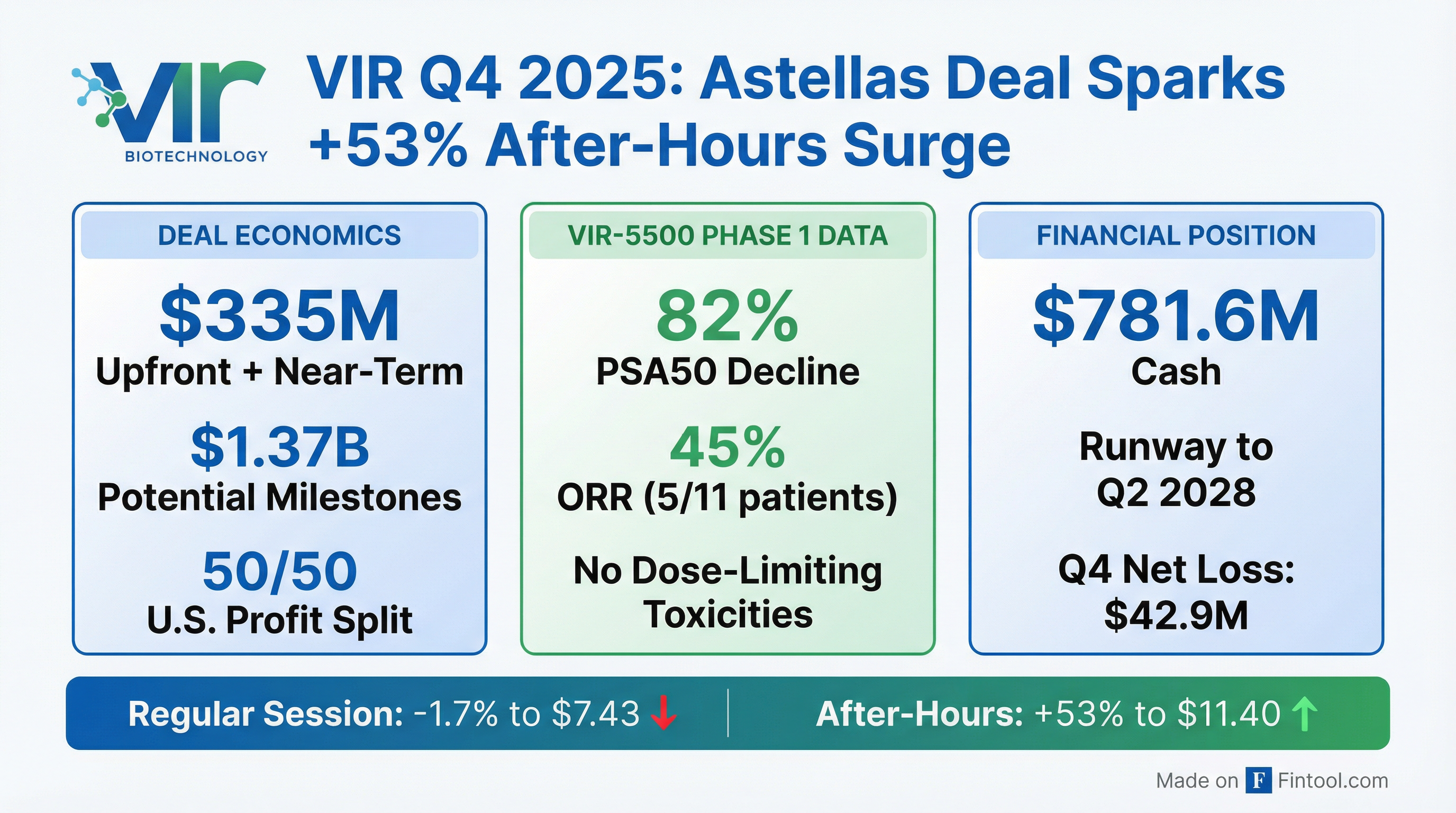
- Vir Biotechnology's stock jumped about 55% after announcing a major collaboration with Astellas to co-develop VIR-5500, which includes $335 million upfront and over $1.37 billion in potential additional milestones and royalties.
- Positive Phase 1 data for VIR-5500 showed encouraging anti-tumor activity, with PSA50 declines in about 82% of evaluable patients and objective responses in 45%.
- The company reported a Q4 revenue beat of roughly $64.1 million and an EPS result better than estimates.
- Vir's annual 10-K disclosed full-year revenue of $68.6 million and a sizeable net loss ($438 million), though operating losses improved year-over-year.
- Cash, cash equivalents, and investments are expected to fund operations into the second quarter of 2028.
- Vir Biotechnology announced a strategic collaboration with Astellas for the global development and commercialization of VIR-5500, a PRO-XTEN dual-masked PSMA-targeting T-cell engager for prostate cancer.
- The collaboration includes $335 million in combined upfront and near-term payments, comprising $240 million in cash and a $75 million equity investment, with a total potential of $1.7 billion in combined upfront and milestone payments.
- New Phase I data for VIR-5500, to be presented at ASCO GU, shows a favorable safety and efficacy profile and dose-dependent antitumor activity in heavily pretreated metastatic castration-resistant prostate cancer patients.
- For 2025, the company reported a net loss of $438 million, with R&D expenses decreasing 10% to $456 million and SG&A expenses decreasing 23% to $92 million compared to 2024.
- Vir Biotechnology ended 2025 with approximately $782 million in cash, cash equivalents, and investments, and anticipates a cash runway extending into the second quarter of 2028.
- Vir Biotechnology reported a net loss of $(438.0) million for 2025, an improvement from $(522.0) million in 2024, with total revenues of $68.6 million.
- The company concluded 2025 with $782 million in cash and cash equivalents, providing a projected cash runway into Q2 2028.
- The Phase 1 study of VIR-5500 for metastatic castration-resistant prostate cancer (mCRPC) demonstrated a favorable safety profile with no dose-limiting toxicities and 12% Grade ≥ 3 treatment-related adverse events.
- VIR-5500 exhibited meaningful anti-tumor activity, including a 45% Objective Response Rate in RECIST-evaluable patients at higher doses and an 82% PSA50 response.
- Vir Biotechnology announced a strategic collaboration with Astellas for the global development and commercialization of VIR-5500, a T-cell engager for prostate cancer.
- The collaboration includes $335 million in combined upfront and near-term payments, with potential milestones up to $1.37 billion, totaling $1.7 billion.
- New Phase I data for VIR-5500 demonstrated a favorable safety and efficacy profile in advanced metastatic castration-resistant prostate cancer, including no dose-limiting toxicities and clear dose-response relationships.
- For 2025, the company reported a net loss of $438 million, with R&D expenses at $456 million (down 10% from 2024) and SG&A expenses at $92 million (down 23% from 2024).
- Vir Biotechnology ended 2025 with approximately $782 million in cash, cash equivalents, and investments, extending its cash runway into the second quarter of 2028.
- Vir Biotechnology announced a strategic collaboration with Astellas for the global development and commercialization of its VIR-5500 T-cell engager, with potential payments up to $1.7 billion, including $335 million in combined upfront and near-term payments.
- The company reported positive Phase I data for VIR-5500, demonstrating a favorable safety and efficacy profile with no dose-limiting toxicities and predominantly low-grade cytokine release syndrome events in metastatic castration-resistant prostate cancer patients.
- For 2025, Vir Biotechnology reported a net loss of $438 million, with R&D expenses decreasing by 10% to $456 million and SG&A expenses decreasing by 23% to $92 million compared to 2024.
- The company ended 2025 with approximately $782 million in cash, cash equivalents, and investments, projecting a cash runway into the second quarter of 2028.
- Vir Biotechnology, Inc. (VIR) and Astellas Pharma Inc. announced a global strategic collaboration to co-develop and co-commercialize VIR-5500, an investigational PSMA-targeting PRO-XTEN® dual-masked T-cell engager for the treatment of prostate cancer.
- Under the terms of the agreement, Vir Biotechnology will receive $335 million in upfront and near-term payments, including $240 million in cash, and is eligible for up to an additional $1.37 billion in development, regulatory, and sales milestones, along with tiered, double-digit royalties on ex-U.S. net sales.
- Global development costs for VIR-5500 will be shared, with Astellas responsible for 60% and Vir Biotechnology for 40%, while U.S. profit/loss will be split 50/50.
- VIR-5500 is currently in Phase 1 development and has shown a favorable safety profile and promising anti-tumor activity in updated data. Vir Biotechnology anticipates initiating dose-expansion cohorts in Q2 2026 and pivotal Phase 3 trials in 2027.
Fintool News
In-depth analysis and coverage of Vir Biotechnology.
Quarterly earnings call transcripts for Vir Biotechnology.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more