Earnings summaries and quarterly performance for REGENERON PHARMACEUTICALS.
Executive leadership at REGENERON PHARMACEUTICALS.
Leonard Schleifer
President and Chief Executive Officer
Andrew Murphy
Executive Vice President, Research
Christopher Fenimore
Executive Vice President, Finance and Chief Financial Officer
Daniel Van Plew
Executive Vice President and General Manager, Industrial Operations and Product Supply
George Yancopoulos
President and Chief Scientific Officer
Jason Pitofsky
Vice President, Controller
Joseph LaRosa
Executive Vice President, General Counsel and Secretary
Marion McCourt
Executive Vice President, Commercial
Board of directors at REGENERON PHARMACEUTICALS.
Arthur Ryan
Director
Bonnie Bassler
Director
Christine Poon
Lead Independent Director
Craig Thompson
Director
David Schenkein
Director
George Sing
Director
Huda Zoghbi
Director
Joseph Goldstein
Director
Kathryn Guarini
Director
Michael Brown
Director
N. Anthony Coles
Director
Research analysts who have asked questions during REGENERON PHARMACEUTICALS earnings calls.
Salveen Richter
Goldman Sachs
9 questions for REGN
Tyler Van Buren
TD Cowen
9 questions for REGN
Akash Tewari
Jefferies
8 questions for REGN
Evan Seigerman
BMO Capital Markets
8 questions for REGN
Terence Flynn
Morgan Stanley
8 questions for REGN
Alexandria Hammond
Wolfe Research
7 questions for REGN
Brian Abrahams
RBC Capital Markets
7 questions for REGN
David Risinger
Leerink Partners
7 questions for REGN
Carter L. Gould
Barclays
6 questions for REGN
Cory Kasimov
Evercore ISI
6 questions for REGN
Christopher Schott
JPMorgan Chase & Co.
5 questions for REGN
Geoff Meacham
Citigroup Inc.
4 questions for REGN
Christopher Raymond
Piper Sandler
3 questions for REGN
William Pickering
Sanford C. Bernstein & Co.
3 questions for REGN
Chris Raymond
Raymond James
2 questions for REGN
Jeffrey Meacham
Citi
2 questions for REGN
Mohit Bansal
Wells Fargo & Company
2 questions for REGN
Simon Goodwin
Rothschild & Co. Redburn
2 questions for REGN
Taylor Hanley
JPMorgan Chase & Co.
2 questions for REGN
Tazeen Ahmad
Bank of America
2 questions for REGN
Tim Anderson
Bank of America
2 questions for REGN
Alice Natelton
Bank of America
1 question for REGN
Chris
Morgan Stanley
1 question for REGN
Trung Huynh
UBS Group AG
1 question for REGN
Recent press releases and 8-K filings for REGN.
- Regeneron will showcase new Phase 3 results for EYLEA HD (aflibercept 8 mg) at the Angiogenesis meeting on February 7, 2026, highlighting its unparalleled durability and comparable efficacy/safety to EYLEA 2 mg with fewer injections.
- Data from the QUASAR trial will include final long-term outcomes through 64 weeks, confirming the first every-two-month treatment option for RVO patients who previously required monthly injections.
- ELARA Phase 3b interim data will be presented, showing that patients switched to monthly EYLEA HD from other anti-VEGF therapies experienced improved visual acuity and better anatomic control.
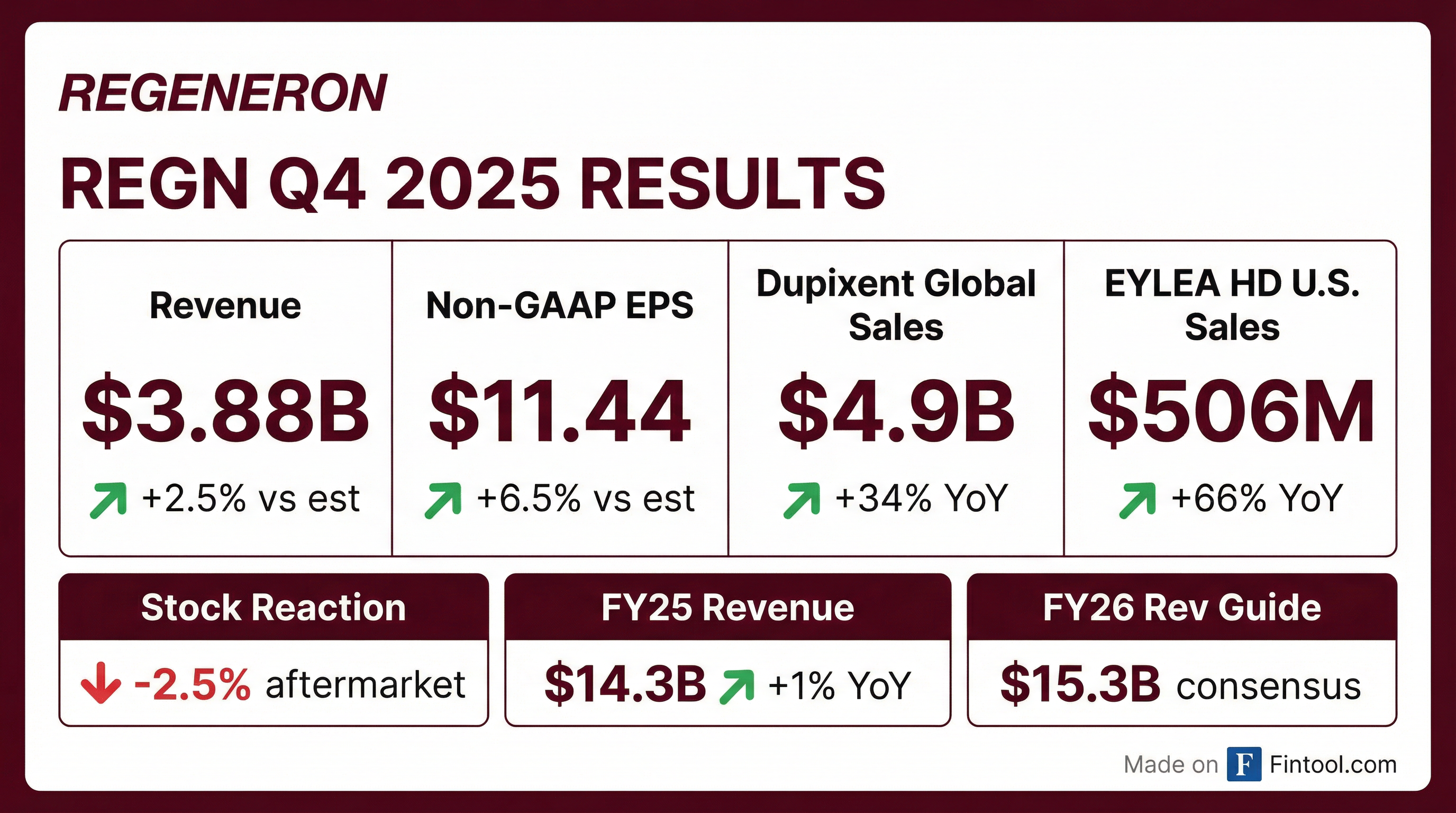
- Q4 total revenues of $3.9 billion, up 3% year-over-year; diluted EPS of $11.44 on net income of $1.2 billion.
- Key product sales: Libtayo net sales of $425 million; EYLEA HD net sales of $506 million (combined U.S. EYLEA franchise of $1.1 billion); DUPIXENT global net sales of $4.9 billion.
- Generated $4.1 billion in free cash flow; cash and marketable securities less debt of $16.2 billion; returned $3.8 billion to shareholders (including $3.4 billion in share repurchases) and declared a quarterly dividend of $0.94 per share (annualized $3.76).
- 2026 guidance: R&D spend of $5.9–6.1 billion; SG&A of $2.5–2.65 billion; gross margin 83–84%; capex $1.1–1.3 billion; effective tax rate 13–15%.
- Regeneron posted Q4 2025 total revenues of $3.9 B and non-GAAP EPS of $11.44.
- Dupixent net sales of $4.9 B in 4Q25 (+32% YoY), with >1.4 M patients on therapy; approvals in CSU (EU) and pediatric asthma (Japan) broaden label.
- LIBTAYO sales rose 13% YoY to $425 M in Q4, driven by adjuvant CSCC and NSCLC uptake.
- Key pipeline milestones: sBLA priority review for AFRS (PDUFA Feb 2026), BLA submission for DB-OTO in genetic hearing loss (decision 1H 2026), garetosmab FOP submissions.
- Q4 2025 total revenue was $3.9 billion, up 3% year-over-year; diluted EPS of $11.44 on net income of $1.2 billion.
- Key product sales in Q4 2025: Dupixent $4.9 billion (+32% YoY), EYLEA HD US net sales $506 million (+66%), Libtayo $425 million (+13%).
- Generated $4.1 billion free cash flow in 2025 and returned $3.8 billion to shareholders via $3.4 billion in share repurchases (with $1.5 billion remaining authorization) and a $0.94/share quarterly dividend.
- 2026 guidance: R&D spend of $5.9–6.1 billion, SG&A $2.5–2.65 billion, gross margin 83–84%, CapEx $1.1–1.3 billion, and effective tax rate 13–15%.
- Pipeline outlook includes at least four FDA approvals (three new molecular entities plus EYLEA HD prefilled syringe) and initiation of 18 Phase 3 studies (targeting ~35,000 patients).
- Regeneron delivered $3.9 billion in Q4 2025 revenues, up 3% year-over-year, with non-GAAP diluted EPS of $11.44.
- Dupixent Q4 net sales reached $4.9 billion (+32% yoy), Eylea HD US net sales were $506 million (+66% yoy), and Libtayo net sales were $425 million (+13% yoy).
- 2026 guidance expects R&D spend of $5.9 billion–$6.1 billion and SG&A of $2.5 billion–$2.65 billion.
- Returned $3.8 billion to shareholders in 2025 (share repurchases of $3.4 billion, dividends of $400 million) and initiated a quarterly dividend of $0.94 per share.
- Fourth quarter 2025 revenues increased 3% to $3.9 billion, and full year revenues rose 1% to $14.3 billion; Q4 GAAP EPS was $7.86 and non-GAAP EPS $11.44, while FY GAAP EPS was $41.48 and non-GAAP EPS $44.31
- Dupixent global net sales reached $4.9 billion in Q4 (+34%) and $17.8 billion for the year (+26%); EYLEA HD U.S. net sales grew 66% to $506 million in Q4
- The company repurchased $671 million of stock in Q4 and $3.5 billion in FY 2025, with $1.5 billion remaining under its buyback program
- 2026 guidance includes GAAP R&D of $6.45 billion–$6.68 billion, GAAP gross margin on net product sales of 79%–80%, and capital expenditures of $1.1 billion–$1.3 billion
- A cash dividend of $0.94 per share was declared, payable March 5, 2026
- Achieved Q4 2025 revenues of $3.884 billion (+3% YoY) and full year 2025 revenues of $14.343 billion (+1%)
- Dupixent global net sales rose 34% to $4.9 billion in Q4 and 26% to $17.8 billion for the full year
- Posted GAAP EPS of $7.86 and non-GAAP EPS of $11.44 in Q4 2025
- Returned capital with $3.5 billion of share repurchases in FY 2025 and declared a $0.94/share dividend payable March 5, 2026
- Global market to rise from USD 3.24 Billion in 2025 to USD 6.93 Billion by 2031 at a 13.5% CAGR
- Expansion driven by reduced sequencing costs via high-throughput systems (e.g., Illumina shipped 352 NovaSeq X units in 2023) and large public programs (NIH’s All of Us released 245,388 genomes)
- Regeneron’s genetics center sequenced its two millionth exome in 2024, illustrating rapid data growth in the industry
- Key challenges include computational/storage bottlenecks and data governance issues: 54% of experts cite unstructured data and 48% note lack of metadata standardization
- Market trends favor long-read sequencing (PacBio shipped 173 Revio systems in FY 2023) and increased adoption of whole exome sequencing in oncology (Guardant Health test volumes up 20% YoY to 46,900)
- Platform and pipeline: Regeneron’s proprietary platforms have yielded 45 clinical candidates across six therapeutic areas and 14 internally discovered approvals, averaging one approval per year over the past 15 years.
- Eylea HD growth: In Q4 2025, U.S. net sales of Eylea HD were $506 million, up 66% YoY and 18% sequentially, with label expansion and an FDA filler decision expected in Q2 2026 to support further uptake.
- Dupixent performance: Dupixent now treats over 1.3 million patients, with annualized global net sales exceeding $19 billion as of Q3 2025, growing 27% YoY.
- Key upcoming readouts: Phase 3 data anticipated H1 2026 include the fianlimab + Libtayo melanoma trial , linvoseltamab’s broad MRD negativity in multiple myeloma , and pivotal cemdisiran + pozelimab results in PNH and myasthenia gravis.
- Strategic investments and innovations: Regeneron plans $6 billion in R&D and $7 billion in capital investments for 2026; advancing novel programs such as an OLA + Praluent obesity combo, DB-OTO gene therapy for deafness, and Garetosumab for FOP with potential approvals in 2026.
- Regeneron highlighted its science-first, big data-driven strategy, leveraging proprietary platforms (VelocImmune, VelociAb) to support a pipeline of 45 clinical candidates across six therapeutic areas and 14 internally discovered approvals over the past 15 years.
- In Q4 2025, the Retina franchise generated $1.1 billion in U.S. net sales (Eylea HD + Eylea), with Eylea HD at $506 million (+66% YoY, +18% QoQ), while Dupixent reached >1.3 million active patients and $19 billion annualized net sales.
- The company plans $6 billion in R&D investment and $7 billion+ in U.S. capital expenditures for 2026, and returned $3.8 billion to shareholders in 2025 via share buybacks and the initiation of a dividend.
- Key upcoming catalysts include pivotal readouts for the fianlimab + Libtayo melanoma combo, linvoseltamab monotherapy in first-line myeloma, cemdisiran/pozelimab in PNH and MG, Factor XI antibody anticoagulants, obesity therapy combinations, and data for gene therapy DB-OTO and Garetosumab in rare diseases.
Quarterly earnings call transcripts for REGENERON PHARMACEUTICALS.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more