Earnings summaries and quarterly performance for BOSTON SCIENTIFIC.
Executive leadership at BOSTON SCIENTIFIC.
Michael Mahoney
President and Chief Executive Officer
Arthur Butcher
Executive Vice President and Group President, MedSurg and Asia Pacific
Daniel Brennan
Executive Vice President and Chief Financial Officer
Jeffrey Mirviss
Executive Vice President and President, Peripheral Interventions
John Sorenson
Executive Vice President, Global Operations
Joseph Fitzgerald
Executive Vice President and Group President, Cardiology
Miriam O'Sullivan
Senior Vice President, Chief Human Resources Officer
Vance Brown
Senior Vice President, General Counsel and Corporate Secretary
Board of directors at BOSTON SCIENTIFIC.
Research analysts who have asked questions during BOSTON SCIENTIFIC earnings calls.
Danielle Antalffy
UBS Group AG
8 questions for BSX
David Roman
Goldman Sachs Group Inc.
8 questions for BSX
Travis Steed
Bank of America
8 questions for BSX
Patrick Wood
Morgan Stanley
7 questions for BSX
Robert Marcus
JPMorgan Chase & Co.
6 questions for BSX
Frederick Wise
Stifel
5 questions for BSX
Joanne Wuensch
Citigroup Inc.
5 questions for BSX
Joshua Jennings
TD Cowen
5 questions for BSX
Larry Biegelsen
Wells Fargo & Company
5 questions for BSX
Michael Polark
Wolfe Research
5 questions for BSX
Vijay Kumar
Evercore ISI
5 questions for BSX
Chris Pasquale
Nephron Research LLC
4 questions for BSX
Lawrence Biegelsen
Wells Fargo
3 questions for BSX
Rick Wise
Stifel Financial Corp
3 questions for BSX
Joanne Lynch
Citigroup Inc.
2 questions for BSX
Matthew Miksic
Barclays PLC
2 questions for BSX
Pito Chickering
Deutsche Bank
2 questions for BSX
Robbie Marcus
JPMorgan Chase & Co.
2 questions for BSX
Anthony Petrone
Mizuho Group
1 question for BSX
Christopher Pasquale
Nephron Research
1 question for BSX
Danielle Antalffy
UBS
1 question for BSX
Joanne Winch
Citibank
1 question for BSX
Marie Thibault
BTIG
1 question for BSX
Matthew O'Brien
Piper Sandler & Co.
1 question for BSX
Matthew Taylor
Jefferies
1 question for BSX
Matt O'Brien
Piper Sandler Companies
1 question for BSX
Matt Taylor
Jefferies & Company Inc.
1 question for BSX
Michael Pollock
Wolfe Research, LLC
1 question for BSX
Mike Polark
Wolfe Research, LLC
1 question for BSX
Peter Chickering
Deutsche Bank AG
1 question for BSX
Recent press releases and 8-K filings for BSX.
- Boston Scientific entered into a $6.0 billion Term Loan Credit Agreement dated February 26, 2026 to finance, in part, its acquisition of Penumbra, Inc.
- The company also established a $3.0 billion Credit Agreement for revolving and multicurrency loans to fund working capital and general corporate needs
- Additionally, Boston Scientific put in place a $2.0 billion 364-day Credit Agreement providing short-term revolving and multicurrency commitments
- Boston Scientific’s Board increased from ten to twelve members and appointed Cathy Smith and Christophe Weber as directors, effective February 18, 2026.
- Smith brings extensive expertise in finance and corporate strategy from roles at Starbucks, Nordstrom and Walmart; Weber contributes global pharmaceutical leadership as Takeda’s retiring CEO (June 2026).
- Under the non-employee director compensation program, each new director will receive a prorated $24,663 cash retainer and an equity award valued at $42,420, vesting at the end of their term.
- In a separate action, the Board approved raising its stock repurchase authorization by $4.0 billion, bringing the total repurchase capacity to $5.0 billion, all fully available as of February 18, 2026.
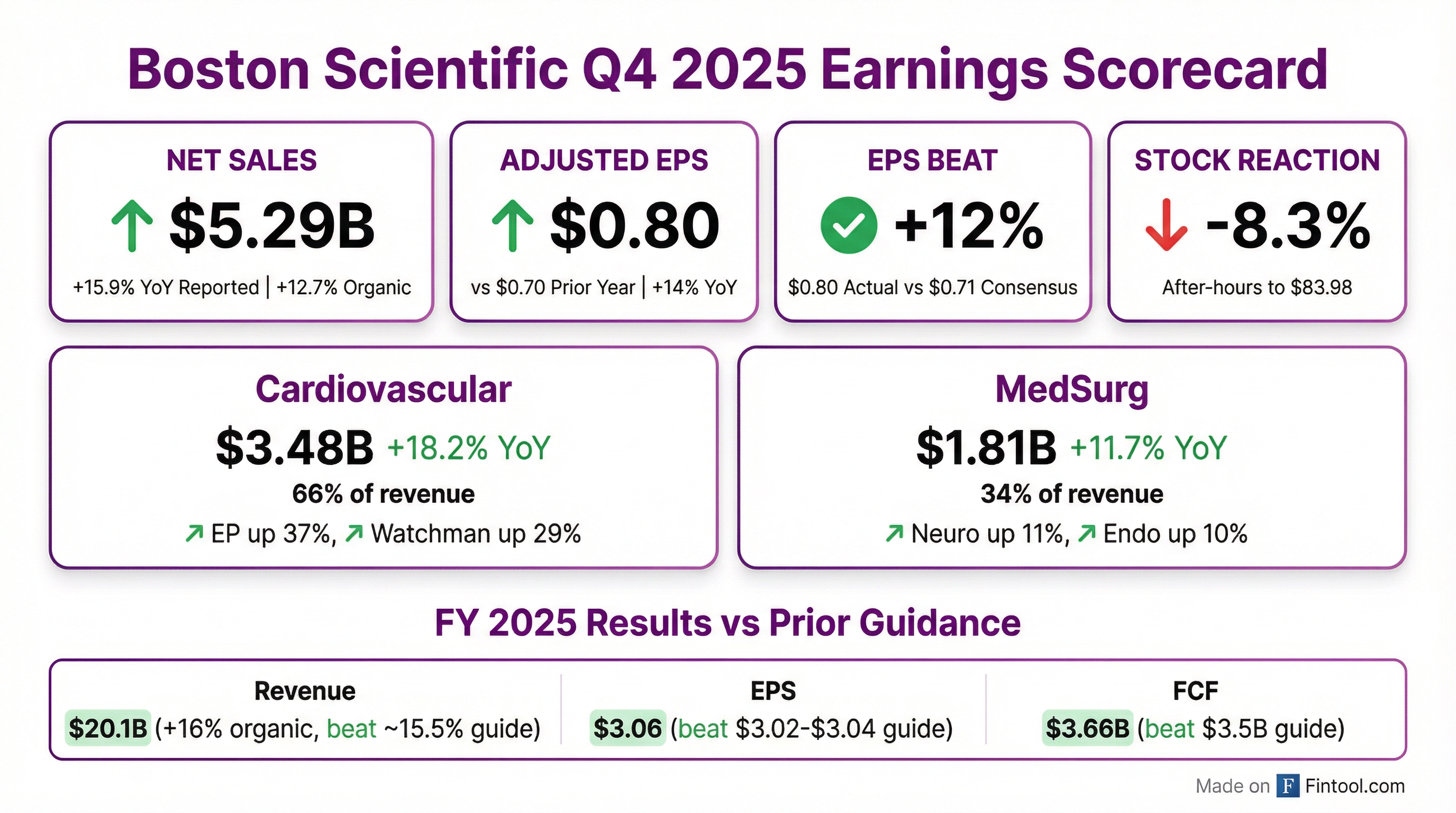
- Q4 2025 consolidated revenue of $5.286 billion, up 15.9% reported (14.3% operational) and 12.7% organic; Q4 adjusted EPS of $0.80, up 15%, exceeding the high end of guidance.
- Full-year 2025 revenue of $20.074 billion, up 19.9% reported (19.2% operational) and 15.8% organic; full-year adjusted EPS of $3.06, up 22%, surpassing guidance.
- Q4 adjusted gross margin of 70.7% and operating margin of 27.3%; full-year adjusted operating margin expanded 100 bps to 28.0%.
- 2026 guidance: full-year organic revenue growth of 10%-11%, adjusted EPS of $3.43–$3.49, and adjusted operating margin expansion of 50–75 bps; Q1 2026 organic growth of 8.5%–10% with adjusted EPS of $0.78–$0.80.
- Q4 2025 consolidated revenue of $5.286 bn (+15.9% y/y; 12.7% organic) and adjusted EPS of $0.80 (+15%).
- Full year 2025 revenue of $20.074 bn (+19.9% y/y; 15.8% organic) and adjusted EPS of $3.06 (+22%).
- Q4 adjusted gross margin 70.7%, operating margin 27.3%; FY adjusted gross margin 70.6% (+30 bps) and operating margin 28.0% (+100 bps).
- 2026 guidance: Q1 organic revenue growth 8.5–10% with EPS $0.78–$0.80; full-year organic growth 10–11% with EPS $3.43–$3.49.
- In Q4 2025, net sales rose 15.9% to $5.286 B, with 12.7% organic growth year-over-year.
- Reported EPS was $0.45 (adjusted $0.80) vs. $0.38 (adj. $0.70) in Q4 2024.
- Free cash flow was $1.013 B vs. $1.181 B in Q4 2024.
- Segment performance: Watchman +29.4%, Electrophysiology +37.1%, Cardiovascular +18.2% Y/Y.
- Q1 2026 guidance: net sales growth 10.5%–12.0% (organic 8.5%–10.0%), adj. EPS $0.78–$0.80; FY 2026: net sales +10.5%–11.5%, organic +10.0%–11.0%, adj. EPS $3.43–$3.49.
- Q4 2025 consolidated revenue of $5.286 billion (+15.9% reported, +14.3% operational, +12.7% organic) and adjusted EPS of $0.80 (+15%); adjusted gross margin 70.7% and operating margin 27.3%.
- Full-year 2025 revenue of $20.074 billion (+19.9% reported, +19.2% operational, +15.8% organic) with adjusted EPS of $3.06 (+22%); free cash flow $3.659 billion (38% growth, 80% conversion) and 28.0% operating margin (+100 bps).
- 2026 guidance: organic revenue growth of 10%–11%, adjusted operating margin expansion of 50–75 bps, and adjusted EPS of $3.43–3.49 (+12%–14%); Q1 2026 organic growth of 8.5%–10% with EPS of $0.78–0.80.
- Business highlights: EP grew 35% in Q4 (market ~18%–20%), Watchman grew 29%, with strong Asia Pac (+15%) and MEA (+5%) performances; Urology and Endoscopy growth tempered by product discontinuations.
- Capital deployment: 2026 free cash flow projected at $4.2 billion; closed Nalu Medical acquisition, pending Valencia and Penumbra deals; prioritizing strategic tuck-in M&A and share repurchases.
- Q4 net sales were $5.286 billion, up 15.9% on a reported basis and 12.7% on an organic basis year-over-year.
- Q4 GAAP EPS was $0.45, versus $0.38 a year ago; adjusted EPS was $0.80, up from $0.70.
- FY 2025 net sales reached $20.074 billion, up 19.9% reported and 15.8% organic; full-year GAAP EPS was $1.94, with adjusted EPS of $3.06.
- 2026 guidance calls for net sales growth of 10.5–11.5% reported (organic 10.0–11.0%) and adjusted EPS of $3.43–3.49; Q1 2026 growth is pegged at 10.5–12.0% reported (organic 8.5–10.0%) with adjusted EPS of $0.78–0.80.
- Q4 net sales of $5.286 billion, up 15.9% reported year-over-year; GAAP net income of $672 million (EPS $0.45), adjusted EPS $0.80 per share.
- Full year net sales of $20.074 billion, up 19.9% reported; GAAP net income of $2.898 billion (EPS $1.94), adjusted EPS $3.06 per share.
- Full year 2026 guidance: reported net sales growth expected at 10.5–11.5%, adjusted EPS projected at $3.43–$3.49; Q1 2026 sales growth guidance 10.5–12.0%, adjusted EPS $0.78–$0.80.
- Global cardiovascular devices market was valued at US$74.58 billion in 2025 and is forecast to grow at a 7.70% CAGR to US$157.32 billion by 2035.
- Therapeutic & surgical devices accounted for 77.5% of market revenue in 2025, driven by premium implantable technologies like CRM systems and structural heart implants.
- Boston Scientific’s Farapulse pulsed field ablation system generated over US$1 billion in 2024, highlighting rapid adoption of PFA over thermal ablation.
- Clinical research remains robust with 51 major cardiovascular trials between 2019–2024 enrolling 292,985 patients, signaling sustained R&D investment.
- North America led the market with a 45.68% revenue share in 2025, underscoring regional dominance in device adoption.
- On January 14, 2026, Boston Scientific entered into a merger agreement to acquire Penumbra, Inc., with Penumbra becoming a wholly owned subsidiary at closing.
- Penumbra shareholders may elect to receive 3.8721 Boston Scientific shares per Penumbra share or $374.00 in cash, with elections prorated to target 73.26% cash and 26.74% stock consideration.
- Outstanding Penumbra options and RSUs will be cancelled or assumed and converted into cash and Boston Scientific stock or RSUs under specified terms, including acceleration for certain vested awards.
- The merger is subject to customary closing conditions, including Penumbra stockholder approval, regulatory clearances and the effectiveness of a Form S-4 registration statement.
Fintool News
In-depth analysis and coverage of BOSTON SCIENTIFIC.
Quarterly earnings call transcripts for BOSTON SCIENTIFIC.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more