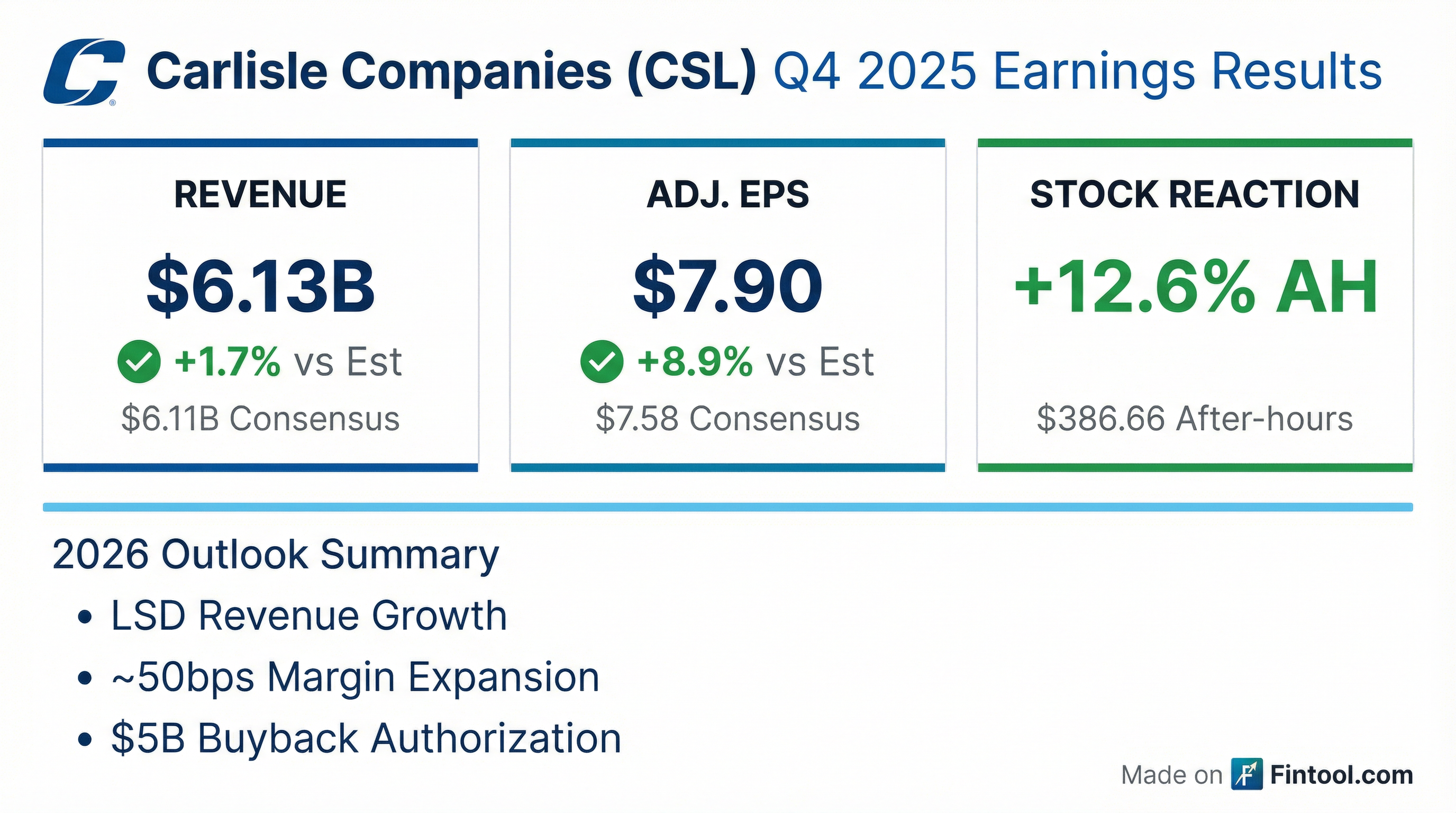
Earnings summaries and quarterly performance for CARLISLE COMPANIES.
Executive leadership at CARLISLE COMPANIES.
D. Christian Koch
Chair, President and Chief Executive Officer
Christopher B. Gaskill
Vice President and General Counsel
Frank J. Ready
President, Carlisle Weatherproofing Technologies
Jason Taylor
President, Carlisle Construction Materials
Kevin P. Zdimal
Vice President and Chief Financial Officer
Scott C. Selbach
Executive Vice President, Government Relations and Secretary
Board of directors at CARLISLE COMPANIES.
Research analysts who have asked questions during CARLISLE COMPANIES earnings calls.
Garik Shmois
Loop Capital Markets
8 questions for CSL
Bryan Blair
Oppenheimer
6 questions for CSL
Keith Hughes
Truist Financial Corporation
6 questions for CSL
Susan Maklari
Goldman Sachs Group Inc.
6 questions for CSL
Adam Baumgarten
Zelman & Associates
5 questions for CSL
David Macgregor
Longbow Research
5 questions for CSL
Timothy Wojs
Robert W. Baird & Co.
4 questions for CSL
Joseph Nolan
Longbow Research
3 questions for CSL
Tomohiko Sano
JPMorgan Chase & Co.
3 questions for CSL
Charles Perron-Piché
Goldman Sachs
2 questions for CSL
Saree Boroditsky
Jefferies
2 questions for CSL
Tim Wojs
Robert W. Baird & Co. Incorporated
2 questions for CSL
Tom Sano
JPMorgan Chase & Co.
2 questions for CSL
Recent press releases and 8-K filings for CSL.
- For the full year 2025, Carlisle delivered $5 billion in revenue, $19.40 adjusted EPS, 24.4% adjusted EBITDA margins, and 25% ROIC. The company generated $972 million in free cash flow, representing a 19.4% margin.
- In the fourth quarter of 2025, revenue was approximately $1.1 billion, adjusted EPS was $3.90, and adjusted EBITDA margin was 22.1%. Organic revenue declined 3% due to soft new construction activity, partially offset by solid commercial reroofing demand.
- Carlisle returned $1.3 billion to shareholders through share repurchases and $181 million through dividends in 2025. For 2026, the company expects low single-digit consolidated revenue growth and approximately 50 basis points of adjusted EBITDA margin expansion.
- The company reaffirmed its Vision 2030 targets of $40 adjusted EPS and more than 25% ROIC, with goals of 30%+ EBITDA margins at CCM and 25%+ at CWT.
- Carlisle reported full-year 2025 revenue of $5 billion and adjusted EPS of $19.40, with adjusted EBITDA margins of 24.4% and ROIC of approximately 25%. For Q4 2025, revenue was $1.1 billion and adjusted EPS was $3.90.
- The company generated $972 million in free cash flow (19.4% margin) in 2025 and returned nearly $1.5 billion to shareholders, including $1.3 billion in share repurchases and $181 million in dividends.
- For 2026, Carlisle anticipates low single-digit revenue growth and approximately 50 basis points of adjusted EBITDA margin expansion, alongside plans to repurchase $1 billion of shares. The company also reaffirmed its Vision 2030 targets of $40 adjusted EPS and over 25% ROIC.
- The company noted steady reroofing demand, which represents approximately 70% of its CCM business and is expected to grow low to mid-single digits in 2026, while the new commercial construction market remains soft with a projected gradual bottoming out mid-year.
- Carlisle reported $1.1 billion in revenue and $3.90 adjusted EPS for Q4 2025, contributing to $5.0 billion in full-year 2025 revenues and $19.40 adjusted EPS.
- The company generated $1.1 billion in operating cash flow and $972 million in free cash flow for FY 2025, returning $1.5 billion to shareholders through dividends and share buybacks.
- For full year 2026, Carlisle projects low single-digit revenue growth and 50 basis points of Adjusted EBITDA Margin expansion.
- As of December 31, 2025, Carlisle maintained $2.1 billion in total liquidity and a Net Debt to EBITDA ratio of 1.4x.
- For the full year 2025, Carlisle delivered $5 billion in revenue and $19.40 in adjusted EPS, with adjusted EBITDA margins of 24.4% and ROIC of approximately 25%.
- The company generated $972 million in free cash flow (19.4% margin) in 2025 and returned $1.3 billion to shareholders through share repurchases and $181 million through dividends.
- In Q4 2025, Carlisle reported $1.1 billion in revenue and $3.90 in adjusted EPS, with organic revenue declining 3% due to soft new construction activity, partially offset by solid commercial reroofing demand.
- Looking ahead to 2026, Carlisle expects low single-digit consolidated revenue growth and approximately 50 basis points of adjusted EBITDA margin expansion, with plans to repurchase $1 billion of shares. The company reaffirmed its Vision 2030 targets of $40 adjusted EPS and over 25% ROIC.
- Carlisle Companies reported Q4 2025 revenue of $1.1 billion, a 0.4% year-over-year increase, with diluted EPS of $3.19 and adjusted EPS of $3.90.
- For the full year 2025, the company generated $1.1 billion in operating cash flow and repurchased $1.3 billion of shares, including $300 million in Q4 2025.
- The 2026 outlook anticipates low-single-digit revenue growth and approximately 50 basis points of adjusted EBITDA margin expansion, with plans to repurchase up to $1 billion of shares.
- Carlisle Companies reported fourth quarter 2025 revenue of $1.1 billion, an increase of 0.4% year-over-year, with diluted EPS of $3.19 and adjusted EPS of $3.90.
- For the full year 2025, the company generated $1.1 billion in operating cash flow and repurchased $1.3 billion of shares, including $300 million in Q4.
- The 2026 outlook includes low-single-digit revenue growth and approximately 50 basis points of adjusted EBITDA margin expansion.
- Carlisle plans to repurchase up to $1 billion of shares in 2026 and maintains a long-term objective of delivering $40 of adjusted EPS.
- CSL announced five-year (60-month) results from the pivotal Phase 3 HOPE-B study for HEMGENIX, confirming the long-term durability and safety of the one-time gene therapy for adults with hemophilia B.
- The study demonstrated that 94% of patients (51 of 54) remained free from continuous prophylaxis treatment through five years, with mean factor IX activity levels sustained at 36.1% at year five.
- HEMGENIX significantly reduced bleeding rates, with the mean adjusted annualized bleeding rate (ABR) for all bleeds decreasing by approximately 90% (from 4.16 to 0.40) from lead-in to year five.
- The therapy maintained a favorable safety profile, with no serious adverse events related to treatment, and is the only commercially available gene therapy for adults with hemophilia B.
- Carlisle (CSL) reported Q3 2025 revenue of $1.3 billion, an increase of 1% year-over-year, and adjusted EPS of $5.61.
- The company revised its full-year 2025 guidance to anticipate flat revenue year-over-year and an adjusted EBITDA margin decline of approximately 250 basis points.
- Performance was driven by the strength of the underlying CCM business, particularly strong reroofing demand, which was partially offset by ongoing challenges in residential and non-residential new construction markets.
- Carlisle maintained its commitment to capital deployment, repurchasing 800,000 shares for $300 million in Q3, raising its dividend by 10%, and increasing its full-year share buyback target to $1.3 billion.
- The company also issued $1 billion of debt in Q3, maintaining a net debt to EBITDA ratio of 1.4 times as of September 30, 2025.
- CSL reported Q3 2025 revenue of $1,347 million, a 1% year-over-year increase, with Adjusted EPS of $5.61 and an Adjusted EBITDA Margin of 25.9%.
- The company returned $347 million to shareholders in Q3 2025 through dividends and share repurchases, and increased its share buyback target for the year to $1.3 billion.
- CSL revised its full-year 2025 outlook to project flat revenue year-over-year and an Adjusted EBITDA margin compression of 250 basis points from 2024.
- Performance was influenced by M&A contributions and strong commercial re-roofing, which helped to offset lower volumes from soft new construction and residential R&R markets.
- Carlisle Companies Incorporated reported Q3 2025 revenue of $1.3 billion, a 1% year-over-year increase, though organic revenue declined 2%.
- Adjusted EPS was $5.61, down 3% from the prior year, with an adjusted EBITDA margin of 25.9%, a 170 basis point decrease.
- The company revised its full-year 2025 guidance, now expecting consolidated revenue to be flat year-over-year and adjusted EBITDA margin to decline approximately 250 basis points.
- Carlisle increased its full-year 2025 share buyback target to $1.3 billion and announced a 10% dividend increase, its 49th consecutive annual increase.
Quarterly earnings call transcripts for CARLISLE COMPANIES.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more