Earnings summaries and quarterly performance for ILLUMINA.
Executive leadership at ILLUMINA.
Board of directors at ILLUMINA.
Anna Richo
Director
Caroline Dorsa
Director
Frances Arnold
Director
Gary Guthart
Director
Keith Meister
Director
Philip Schiller
Director
Robert Epstein
Director
Scott Gottlieb
Independent Chair of the Board
Scott Ullem
Director
Susan Siegel
Director
Research analysts who have asked questions during ILLUMINA earnings calls.
David Westenberg
Piper Sandler
6 questions for ILMN
Tycho Peterson
Jefferies
6 questions for ILMN
Vijay Kumar
Evercore ISI
6 questions for ILMN
Puneet Souda
Leerink Partners
5 questions for ILMN
Kyle Mikson
Canaccord Genuity
4 questions for ILMN
Conor Noel McNamara
RBC Capital Markets
3 questions for ILMN
Dan Arias
Stifel Financial Corp.
3 questions for ILMN
Daniel Brennan
TD Cowen
3 questions for ILMN
Douglas Schenkel
Wolfe Research, LLC
3 questions for ILMN
Doug Schenkel
Wolfe Research LLC
3 questions for ILMN
Jack Meehan
Nephron Research LLC
3 questions for ILMN
Michael Ryskin
Bank of America Merrill Lynch
3 questions for ILMN
Casey Woodring
JPMorgan Chase & Co.
2 questions for ILMN
Daniel Arias
Stifel, Nicolaus & Company, Incorporated
2 questions for ILMN
Patrick Donnelly
Citi
2 questions for ILMN
Subbu Nambiar
Guggenheim
2 questions for ILMN
Subhalaxmi Nambi
Guggenheim Securities
2 questions for ILMN
Sung Ji Nam
Scotiabank
2 questions for ILMN
Ben Miller
Stephens Inc.
1 question for ILMN
Daniel Leonard
Stifel Financial Corp.
1 question for ILMN
Eve Burstein
Goldman Sachs
1 question for ILMN
Rachel Vatnsdal
JPMorgan Chase & Co.
1 question for ILMN
Rachel Vatnsdal Olson
JPMorgan
1 question for ILMN
Subbu Nambi
Guggenheim Securities
1 question for ILMN
Tejas Savant
Morgan Stanley
1 question for ILMN
Recent press releases and 8-K filings for ILMN.
- Illumina, Inc. (NASDAQ: ILMN) launched TruPath™ Genome on February 24, 2026, a new product designed to provide high-quality, comprehensive whole genome insights for genetic disease.
- TruPath Genome offers unparalleled accuracy and resolution, even in difficult genomic regions, with a simple workflow that generates 16 whole genomes per day and fully phases up to 98% of genes.
- The new technology is a cost-effective solution with a list price of $395 USD, including all consumables and analysis for 30x coverage.
- Broad Clinical Labs is among the first to adopt TruPath Genome, which was previously piloted by over 30 early access customers including GeneDx, Rady Children's Hospital, and Baylor College of Medicine.
- The Next Generation Sequencing (NGS) Market is projected to grow significantly from an estimated US$ 14.79 Billion in 2025 to US$ 56.25 Billion by 2033, demonstrating an 18.17% Compound Annual Growth Rate (CAGR) during this forecast period.
- This market expansion is primarily driven by advancements in sequencing processes, increased applications in genomics research and personalized medicine, and rising investments in biotechnology.
- Illumina Inc. is identified as one of the top companies in the NGS Market by 2033, alongside Thermo Fisher Scientific, Pacific Biosciences, and Roche Ltd..
- The report provides a detailed analysis of Illumina, covering its business model, key personnel, recent developments including Mergers & Acquisitions, partnerships, investments, product pipeline, and revenue analysis.
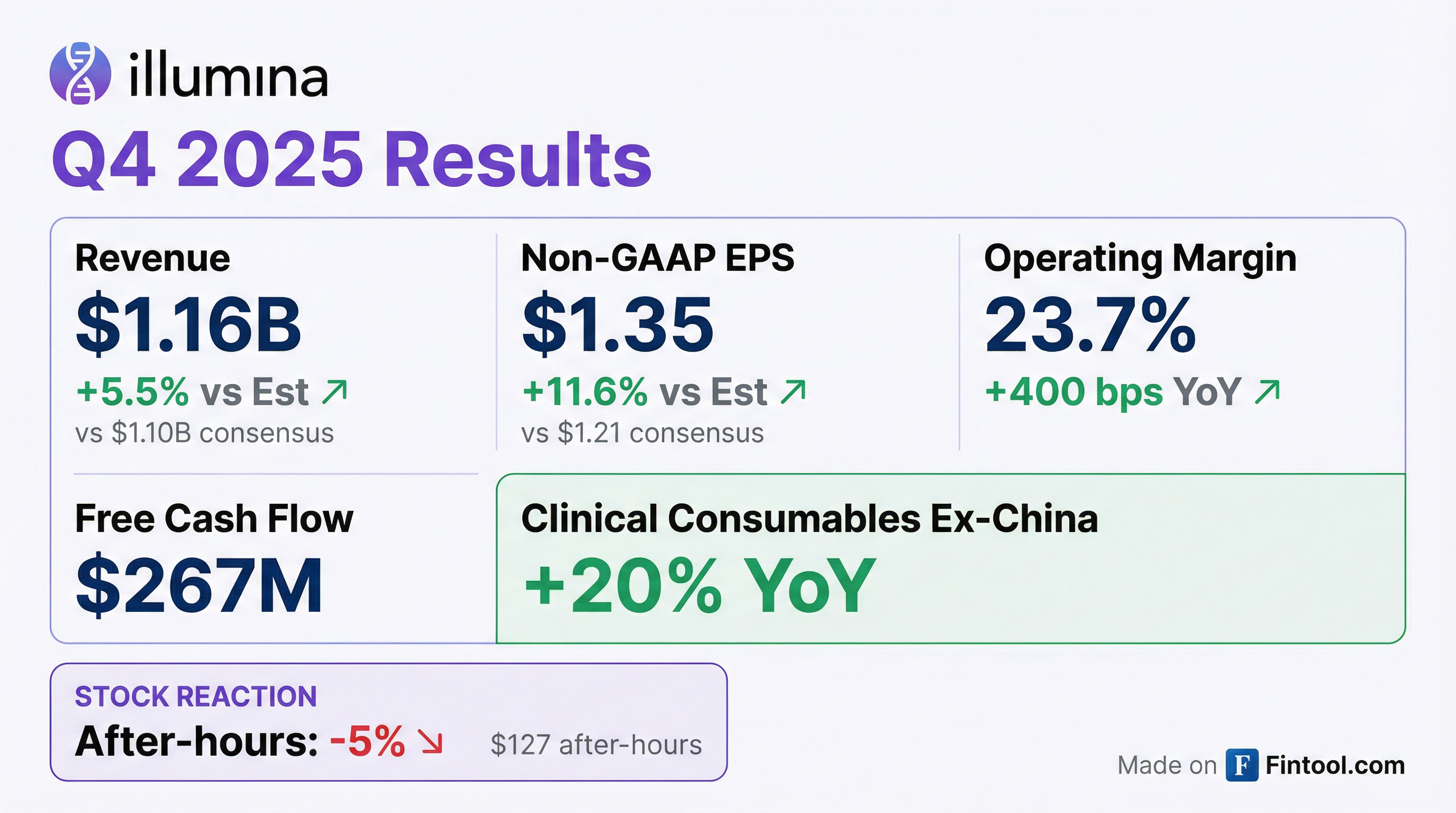
- Illumina reported strong Q4 2025 revenue of $1.16 billion, up 5% year-over-year, driven by 20% growth in clinical consumables revenue ex-China, and non-GAAP EPS of $1.35, up 42% year-over-year. For the full year 2025, the company returned to growth ex-China, expanded non-GAAP operating margins by 180 basis points, and grew non-GAAP EPS by 16% to $4.84.
- For 2026, Illumina expects total company revenue of $4.5-$4.6 billion, representing reported growth of 4%-6%, with organic revenue growth ex-China of 2%-4%. Non-GAAP operating margins are projected to be 23.3%-23.5%, and non-GAAP EPS is guided between $5.05-$5.20, including $0.18 dilution from the SomaLogic acquisition.
- The company completed the acquisition of SomaLogic in January 2026 for an upfront payment of $350 million, strengthening its position in multi-omics. Strategic pillars include core sequencing, scaling multi-omics, and expanding services, data, and software capabilities, highlighted by the launch of BioInsight and the Billion Cell Atlas for drug discovery.
- Illumina generated $931 million in free cash flow for 2025 and returned approximately $740 million to shareholders through share repurchases, with $643 million remaining on its share repurchase authorization.
- Illumina reported Q4 2025 revenue of $1.16 billion, a 5% increase year-over-year (YoY), and non-GAAP diluted EPS of $1.35, a 42% increase YoY. The non-GAAP operating margin for the quarter was 23.7%, up 400 basis points YoY.
- Key growth was driven by Clinical Sequencing Consumables revenue excluding China, which grew 20% YoY in Q4 2025. NovaSeq™ X placements exceeded 100 instruments in Q4 2025, with NovaSeq X accounting for 55% of high-throughput gigabases shipped and 51% of high-throughput consumables revenue.
- The company closed the acquisition of SomaLogic in January 2026 for $350 million cash and up to $75 million in performance-based milestones, expecting SomaLogic to become profitable in 2027 on a non-GAAP operating income basis. For fiscal year 2026, Illumina provided guidance for total revenue between $4.50 billion and $4.60 billion (4% - 6% YoY growth) and non-GAAP diluted EPS between $5.05 and $5.20.
- Illumina's Q4 2025 revenue reached $1.16 billion, marking a 5% year-over-year increase on a reported basis, with non-GAAP EPS of $1.35, representing a 42% growth year-over-year.
- For the full year 2025, the company achieved 2% ex-China revenue growth, expanded non-GAAP operating margins by 180 basis points, and grew non-GAAP EPS by 16% to $4.84.
- The company provided 2026 revenue guidance of $4.5-$4.6 billion, which translates to 4%-6% reported growth, and non-GAAP EPS guidance of $5.05-$5.20, including a $0.18 dilution from the SomaLogic acquisition.
- Key drivers for 2025 performance included 20% ex-China growth in clinical consumables revenue in Q4 and strong NovaSeq X placements, with the acquisition of SomaLogic enhancing multi-omics capabilities.
- Illumina anticipates double-digit to mid-teens clinical growth in 2026, while research and applied consumables revenue is projected to decline mid-to-high single-digits.
- Illumina reported Q4 2025 revenue of $1.16 billion, an increase of 5% year-over-year on a reported basis, with ex-China revenue growing 7%. Non-GAAP EPS for the quarter was $1.35, marking a 42% year-over-year increase.
- For the full year 2025, Illumina achieved 2% ex-China revenue growth, expanded non-GAAP operating margins by 180 basis points, and grew non-GAAP EPS by 16% to $4.84. The company also generated $931 million in free cash flow and returned approximately $740 million to shareholders through share repurchases.
- The company provided 2026 guidance, projecting total revenue of $4.5-$4.6 billion, representing 4%-6% reported growth. Organic revenue growth ex-China is anticipated to be 2%-4%, with clinical consumables expected to grow double-digit to mid-teens.
- Illumina forecasts 2026 non-GAAP EPS between $5.05-$5.20, which includes an $0.18 dilution from the recently completed SomaLogic acquisition. Operating margins are expected to be 23.3%-23.5% in 2026.
- The company remains confident in achieving its 2027 long-term targets of high single-digit growth and a 26% operating margin.
- Illumina reported Q4 2025 revenue of $1.16 billion, a 5% increase from Q4 2024, with non-GAAP diluted EPS of $1.35.
- For fiscal year 2025, the company's revenue was $4.34 billion, flat compared to 2024, and non-GAAP diluted EPS was $4.84.
- The company provided fiscal year 2026 guidance, expecting total company revenue between $4.5 billion and $4.6 billion (4% - 6% growth) and non-GAAP diluted EPS in the range of $5.05 - $5.20.
- Key announcements include the completion of the SomaLogic acquisition and the lifting of the Chinese Ministry of Commerce (MOFCOM) export ban on Illumina sequencers.
- Illumina reported Q4 2025 revenue of $1.16 billion, a 5% increase from Q4 2024, and fiscal year 2025 revenue of $4.34 billion, which was flat compared to 2024.
- Non-GAAP diluted EPS was $1.35 for Q4 2025 and $4.84 for fiscal year 2025.
- For fiscal year 2026, Illumina expects total company revenue of $4.5 billion to $4.6 billion and non-GAAP diluted EPS in the range of $5.05 to $5.20.
- The company completed the SomaLogic acquisition and announced that the Chinese Ministry of Commerce (MOFCOM) lifted the export ban on Illumina sequencers.
- Illumina, Inc. completed its acquisition of SomaLogic on January 30, 2026.
- The acquisition, funded with cash on hand, involved a purchase price of $350 million in cash at closing, plus up to $75 million in near-term performance-based milestones and royalties.
- This deal expands Illumina's multiomics portfolio and strengthens its proteomics capabilities.
- Illumina will discuss the financial implications of this transaction during its upcoming earnings call on February 5, 2026.
- Illumina, Inc. has completed its acquisition of SomaLogic, a leader in data-driven proteomics technology.
- This acquisition expands Illumina's multiomics portfolio, strengthening its capabilities in delivering scalable insights across genomics and proteomics.
- The deal involved $350 million in cash paid at closing, plus up to $75 million in near-term performance-based milestones and royalties, and was funded with cash on hand.
- Illumina will discuss the financial implications of this transaction during its upcoming earnings call scheduled for February 5, 2026.
Fintool News
In-depth analysis and coverage of ILLUMINA.
Quarterly earnings call transcripts for ILLUMINA.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more