Earnings summaries and quarterly performance for AbbVie.
Executive leadership at AbbVie.
Robert A. Michael
Chief Executive Officer
Azita Saleki-Gerhardt
Executive Vice President, Chief Operations Officer
Jeffrey R. Stewart
Executive Vice President, Chief Commercial Officer
Perry C. Siatis
Executive Vice President, General Counsel and Secretary
Scott T. Reents
Executive Vice President, Chief Financial Officer
Board of directors at AbbVie.
Brett J. Hart
Director
Edward J. Rapp
Director
Frederick H. Waddell
Director
Jennifer L. Davis
Director
Melody B. Meyer
Director
Rebecca B. Roberts
Director
Robert J. Alpern
Director
Roxanne S. Austin
Lead Independent Director
Susan E. Quaggin
Director
Thomas C. Freyman
Director
Thomas J. Falk
Director
William H.L. Burnside
Director
Research analysts who have asked questions during AbbVie earnings calls.
Mohit Bansal
Wells Fargo & Company
8 questions for ABBV
Steve Scala
Cowen
8 questions for ABBV
Terence Flynn
Morgan Stanley
8 questions for ABBV
Vamil Divan
Guggenheim Securities
8 questions for ABBV
David Risinger
Leerink Partners
7 questions for ABBV
Christopher Schott
JPMorgan Chase & Co.
6 questions for ABBV
Asad Haider
Goldman Sachs
5 questions for ABBV
Luisa Hector
Berenberg
5 questions for ABBV
Carter L. Gould
Barclays
3 questions for ABBV
Courtney Breen
AllianceBernstein
3 questions for ABBV
David Amsellem
Piper Sandler Companies
3 questions for ABBV
Geoffrey Meacham
Citi
3 questions for ABBV
Trung Huynh
UBS Group AG
3 questions for ABBV
Chris Schott
JPMorgan Chase & Company
2 questions for ABBV
Chris Shibutani
Goldman Sachs Group, Inc.
2 questions for ABBV
Geoff Meacham
Citigroup Inc.
2 questions for ABBV
James Shin
Analyst
2 questions for ABBV
Mark Hoffman
BMO Capital Markets
2 questions for ABBV
Matthew Phipps
William Blair
2 questions for ABBV
Michael Yee
Jefferies
2 questions for ABBV
Simon Baker
Rothschild & Co Redburn
2 questions for ABBV
Timothy Anderson
BofA Securities
2 questions for ABBV
Alexandria Hammond
Wolfe Research
1 question for ABBV
Christopher Raymond
Piper Sandler
1 question for ABBV
David Aslam
Piper Sandler
1 question for ABBV
Evan Seigerman
BMO Capital Markets
1 question for ABBV
Gary Nachman
Raymond James
1 question for ABBV
Jon Win
UBS
1 question for ABBV
Tim Anderson
Bank of America
1 question for ABBV
Recent press releases and 8-K filings for ABBV.
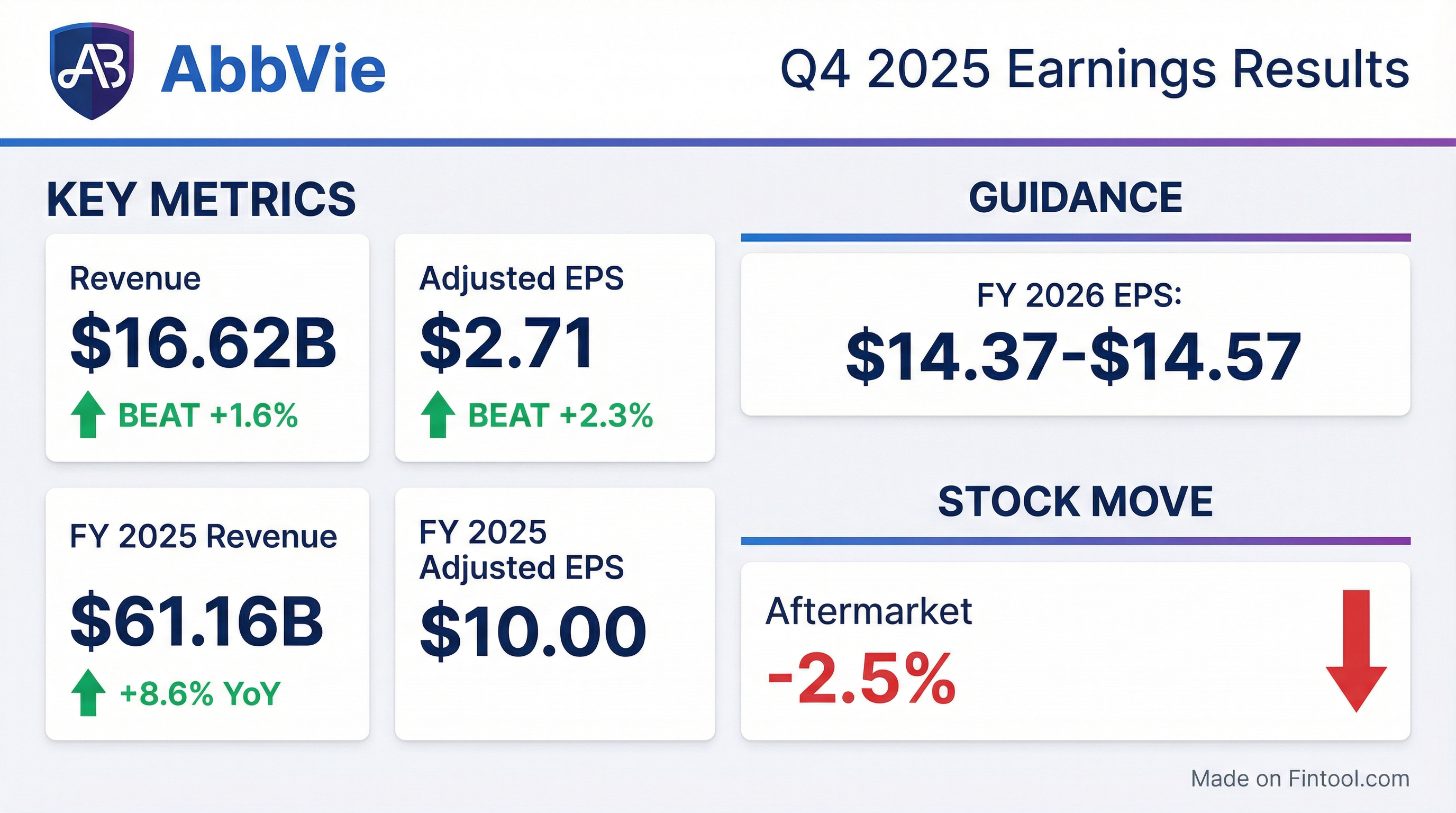
- AbbVie delivered full-year adjusted EPS of $10, beating its guidance midpoint by $0.54 (excluding IPR&D), and achieved record net revenues of $61.2 billion, up 8.6% and $2 billion above guidance despite Humira erosion.
- In Q4 2025, AbbVie reported total net revenues of $16.6 billion, up 10% YoY, and adjusted EPS of $2.71, $0.08 above guidance; the ex-Humira growth platform rose 14.5%, with an adjusted gross margin of 83.6%.
- For 2026, the company forecasts net revenues of ~$67 billion (+9.5%), adjusted EPS of $14.37–14.57, and expects immunology sales of $34.5 billion including Skyrizi at $21.5 billion and Rinvoq at $10.1 billion.
- Immunology performance in Q4 included $8.6 billion in total revenues, with Skyrizi at $5 billion (+31.9%) and Rinvoq at $2.4 billion (+28.6%).
- AbbVie delivered FY 2025 adjusted EPS of $10 and net revenues of $61.2 billion, up 8.6% yoy despite $16 billion of Humira erosion.
- In Q4 2025, AbbVie achieved adjusted EPS of $2.71 and net revenues of $16.6 billion (+10% yoy; ex-Humira platform +14.5%).
- For 2026, AbbVie guides net revenues of ~$67 billion (+9.5% yoy) and adjusted EPS of $14.37–$14.57.
- Immunology franchises delivered Q4 revenues of $8.6 billion, with Skyrizi at $5 billion (+31.9%) and Rinvoq at $2.4 billion (+28.6%); full-year combined revenue reached $25.9 billion (+ > $8 billion yoy).
- AbbVie advanced its pipeline with new approvals (Rinvoq for GCA, Emrelis, Epkinly), funded 90 clinical programs, and invested > $5 billion in business development in 2025.
- Record Q4 net revenues of $16.6 billion and adjusted EPS of $2.71, driving full-year net revenues of $61.2 billion and adjusted EPS of $10.00.
- Skyrizi and Rinvoq combined delivered $25.9 billion in 2025, with Q4 sales of $5 billion and $2.4 billion respectively, while Humira sales declined 26.1% to $1.2 billion in the quarter.
- For 2026, AbbVie guides ~$67 billion in revenues (9.5% growth) and adjusted EPS of $14.37–$14.57, including $34.5 billion in immunology and $12.5 billion in neuroscience sales.
- Continued pipeline investment with $5 billion in business development in 2025 and a three-year US pricing agreement, alongside projected $18.5 billion free cash flow for 2026 to support dividends and BD.
- AbbVie delivered FY 2025 net revenues of $61.160 billion, up 8.6% on a reported basis, and Q4 net revenues of $16.618 billion, up 10.0%.
- GAAP FY 2025 diluted EPS was $2.36 and adjusted diluted EPS was $10.00, including an unfavorable $2.76 per share IPR&D charge; Q4 GAAP EPS was $1.02 and adjusted EPS was $2.71, with a $0.71 IPR&D impact.
- Growth was driven by immunology and neuroscience: FY immunology revenues were $30.406 billion (+14.0%) and Q4 immunology revenues were $8.626 billion (+18.3%); FY neuroscience revenues were $10.767 billion (+19.6%) and Q4 neuroscience revenues were $2.961 billion (+17.9%).
- AbbVie provided 2026 adjusted diluted EPS guidance of $14.37 to $14.57, excluding any IPR&D and milestone impacts.
- AbbVie delivered full-year net revenues of $61.160 B, up 8.6% year-over-year, and reported adjusted diluted EPS of $10.00, down 1.2%.
- In the fourth quarter, net revenues were $16.618 B, a 10.0% increase, with adjusted diluted EPS of $2.71.
- Full-year immunology revenues reached $30.406 B (+14.0%), driven by Skyrizi ($17.562 B) and Rinvoq ($8.304 B); neuroscience revenues were $10.767 B (+19.6%).
- AbbVie provided 2026 adjusted diluted EPS guidance of $14.37–$14.57, excluding acquired IPR&D and milestones expense.
- AbbVie has submitted FDA and EMA applications to expand Rinvoq (upadacitinib) for adults and adolescents with non-segmental vitiligo, supported by Phase 3 Viti-Up trial data showing ≥50% total body and ≥75% facial repigmentation by week 48.
- The filing, if approved, could make Rinvoq the first systemic JAK1-selective therapy for vitiligo, which accounts for 84% of vitiligo cases and represents a significant unmet need.
- AbbVie projects Rinvoq revenue to top $11 billion in 2027, highlighting the indication’s potential to boost its multibillion-dollar franchise amid existing revenues of approximately $59.6 billion and margin pressures.
- AbbVie has filed regulatory applications with the FDA and EMA for upadacitinib 15 mg once daily to treat adult and adolescent non-segmental vitiligo, based on data from the Phase 3 Viti-Up studies.
- In the Viti-Up trials (n=614), upadacitinib achieved the co-primary endpoints of T-VASI 50 and F-VASI 75 at week 48 versus placebo, demonstrating significant re-pigmentation.
- If approved, upadacitinib would be the first systemic therapy for non-segmental vitiligo, addressing a major unmet need in disease stabilization and re-pigmentation.
- US rheumatologists are shifting from broad enthusiasm to more selective, indication-driven expectations for late-stage SLE assets, concentrating interest on a narrower set of perceived leaders.
- Gazyva (obinutuzumab), Rinvoq (upadacitinib), and ianalumab emerge as top late-stage contenders, with Gazyva’s recent lupus nephritis approval expected to reshape treatment pathways, notably in patients with renal involvement.
- Physicians estimate that around 17–20% of moderate-to-severe SLE patients would be appropriate candidates for these leading therapies, reflecting a targeted deployment strategy.
- Interest has moderated for other mid-to-late-stage assets—litifilimab and dapirolizumab pegol face safety and durability concerns, while cenerimod is viewed as a niche oral option.
- Epcoritamab showed improved progression-free survival versus investigator’s choice chemoimmunotherapy in relapsed/refractory DLBCL (PFS HR 0.74 [95% CI 0.60–0.92]).
- The study enrolled 483 patients ineligible for high-dose chemo and stem cell transplant, with benefits in complete response rate, duration of response, and time to next treatment.
- No statistically significant overall survival benefit was observed (OS HR 0.96 [95% CI 0.77–1.20]).
- AbbVie and Genmab will engage global regulators on next steps and present full data at future medical meetings.
- 2026 priorities: focus on operational execution to sustain financial momentum and advance pipeline with expected approvals for Vyalev and Tavapadon, indication expansions for Rinvoq, Ubrelvy, Qulipta, and pivotal data for Lutikizumab and Itentamig.
- Financial outlook: reaffirmed target of high single-digit CAGR through the decade; 2025 top-line grew ~8% with the growth platform expanding ~19%; EPS to outpace revenue via margin expansion and
15% R&D spend ($9 billion). - Immunology: maintain 2027 guidance of $31 billion combined sales for Skyrizi ($20 billion) and Rinvoq ($11 billion), driven by head-to-head trials, market share gains, and low single-digit pricing headwinds.
- Neuroscience & oncology: neuroscience fastest growing segment aiming to be industry leader with Vyalev at ~$450 million in first full year and Tavapadon launch; oncology pipeline advancing ADCs Umbrellas and Teliso, with phase III start for SEZ6-targeting ADC and Itentamig monotherapy ORR readout.
- Aesthetics: investing in consumer outreach to revitalize Botox and filler demand and preparing to launch Trinibot E (short-acting toxin) globally in H2 2026 to lower trial barriers and drive cash-pay growth.
Fintool News
In-depth analysis and coverage of AbbVie.

AbbVie Signs $100 Billion Deal With Trump, Clearing Path for Humira on TrumpRx

AbbVie Pays $650M to Join PD-1/VEGF Bispecific Race in JPM's First Big Deal

AbbVie Nears $20B+ Deal for Revolution Medicines, Capping Historic RAS Rally
Quarterly earnings call transcripts for AbbVie.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more