Earnings summaries and quarterly performance for ELI LILLY &.
Executive leadership at ELI LILLY &.
Board of directors at ELI LILLY &.
Erik Fyrwald
Director
Gabrielle Sulzberger
Director
Jamere Jackson
Director
Jon Moeller
Director
Juan Luciano
Lead Independent Director
Katherine Baicker
Director
Kimberly Johnson
Director
Mary Lynne Hedley
Director
Ralph Alvarez
Director
William Kaelin
Director
Research analysts who have asked questions during ELI LILLY & earnings calls.
Courtney Breen
AllianceBernstein
8 questions for LLY
Evan Seigerman
BMO Capital Markets
8 questions for LLY
Mohit Bansal
Wells Fargo & Company
8 questions for LLY
Seamus Fernandez
Guggenheim Partners
8 questions for LLY
Steve Scala
Cowen
8 questions for LLY
Terence Flynn
Morgan Stanley
8 questions for LLY
Akash Tewari
Jefferies
7 questions for LLY
Asad Haider
Goldman Sachs
6 questions for LLY
Christopher Schott
JPMorgan Chase & Co.
6 questions for LLY
Umer Raffat
Evercore ISI
6 questions for LLY
Alexandria Hammond
Wolfe Research
5 questions for LLY
Geoff Meacham
Citigroup Inc.
5 questions for LLY
David Risinger
Leerink Partners
4 questions for LLY
James Shin
Analyst
4 questions for LLY
Kerry Holford
Berenberg
3 questions for LLY
Tim Anderson
Bank of America
3 questions for LLY
Trung Huynh
UBS Group AG
3 questions for LLY
Alex Hammond
Sidoti & Company, LLC
2 questions for LLY
Chris Schott
JPMorgan Chase & Company
2 questions for LLY
Chris Shibutani
Goldman Sachs Group, Inc.
2 questions for LLY
Geoffrey Meacham
Citi
2 questions for LLY
James Chin
Deutsche Bank
2 questions for LLY
Michael Yee
Jefferies
2 questions for LLY
Timothy Anderson
BofA Securities
2 questions for LLY
Umar Rafat
Evercore
2 questions for LLY
Carter L. Gould
Barclays
1 question for LLY
Jeff Meacham
Citigroup Inc.
1 question for LLY
Kripa Devarakonda
Truist Securities
1 question for LLY
Rajesh Kumar
HSBC
1 question for LLY
Srikripa Devarakonda
Truist Financial Corporation
1 question for LLY
Recent press releases and 8-K filings for LLY.
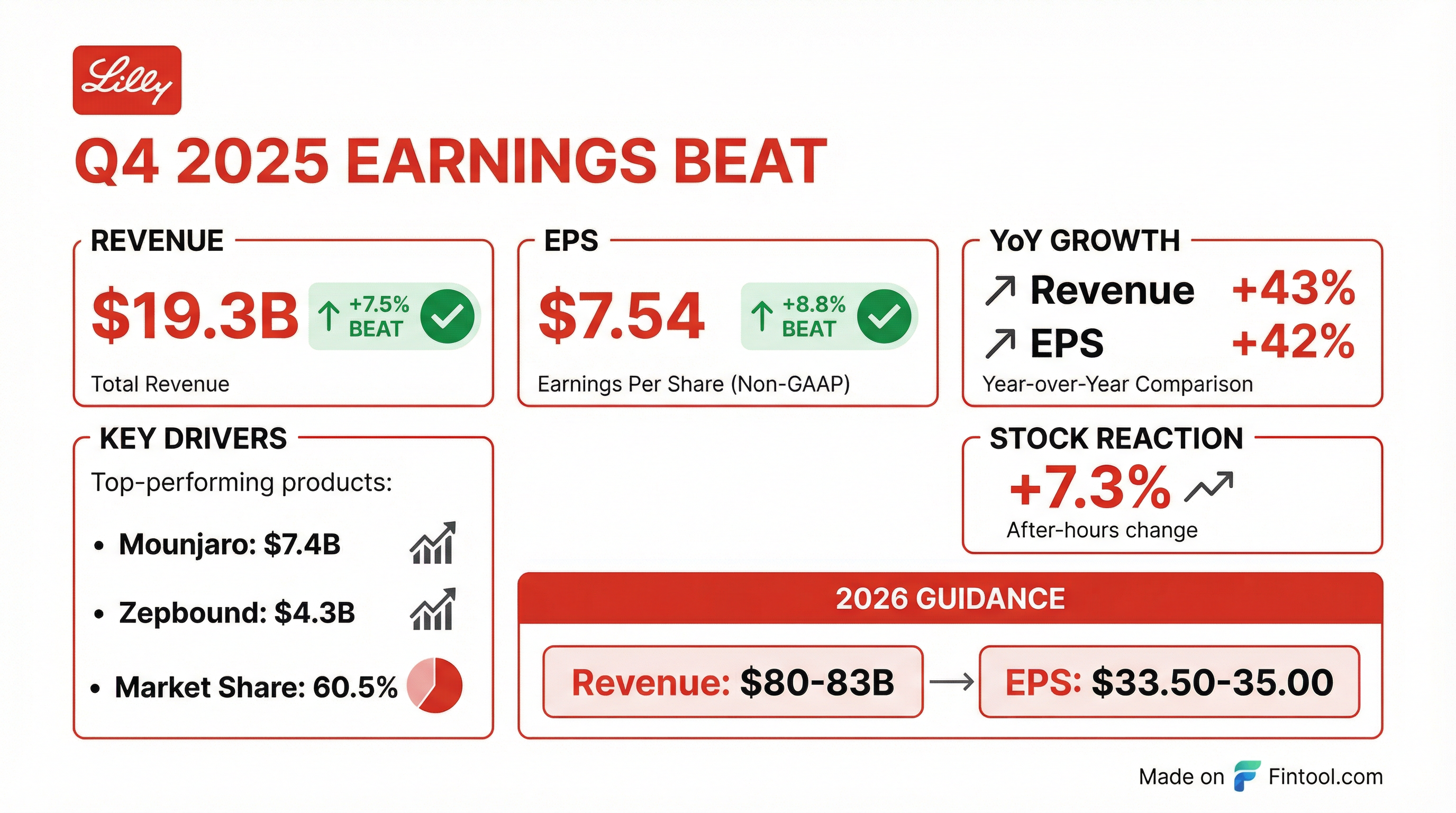
- Lilly’s Q4 revenue rose 43% to $19.3 billion, with adjusted EPS of $7.54, both topping estimates.
- GLP-1 blockbusters Mounjaro and Zepbound drove growth, generating $7.41 billion and $4.26 billion, respectively, in the quarter.
- The company raised its 2026 outlook to $80–$83 billion in revenue and $33.50–35 in adjusted EPS.
- Adjusted operating margins expanded by nearly 300 bps, though the CFO warned pricing pressure will be a headwind.
- Shares jumped 9% to an intraday high of $1,111.08, settling at $1,102.20 on heavy volume.
- Delivered 43% revenue growth to $19.3 billion, driven by incretin analogs with the U.S. market up 33% and Lilly’s share at 60.5%.
- Non-GAAP EPS rose 42% to $7.54.
- R&D spend increased 26% to $3.8 billion, and capital investments jumped 55% to $7.8 billion YTD.
- Returned $1.3 billion in dividends and $1.5 billion in share repurchases.
- Achieved full FDA approval for Jaypirca, pediatric approval for Mounjaro, and reported positive Phase 3 data for retatrutide and orforglipron with submissions in over 40 countries.
- 2025 financial performance: full-year revenue of $65.2 billion (+45% YOY) and EPS of $24.21 (+86% YOY); Q4 revenue grew 43% YOY to support $7.54 EPS
- 2026 guidance update: revenue expected between $80 billion and $83 billion (midpoint +25%); non-GAAP margin of 46–47.5%; EPS forecast $33.50–$35.00
- Capital returns: distributed $1.3 billion in dividends and repurchased $1.5 billion of shares in 2025
- Manufacturing and R&D investments: committed over $55 billion to build out manufacturing since 2020, opened new sites in Wisconsin and North Carolina, and launched an AI co-innovation lab with NVIDIA
- Revenue of $65.2 billion in 2025 (up 45% vs. 2024) and Q4 revenue growth of 43%; EPS of $24.21 for the year (+86%) and Q4 EPS of $7.54.
- 2026 guidance: revenue of $80–83 billion (≈25% midpoint growth), non-GAAP performance margin of 46–47.5%, and EPS of $33.50–35.00.
- Pipeline progress: Orforglipron submitted for obesity in the U.S. and over 40 countries; retatrutide delivered 29% mean body-weight loss at 68 weeks; 36 active Phase III programs and 14 new Phase III trials initiated.
- Capital allocation: $1.3 billion in dividends distributed and $1.5 billion in share repurchases in 2025.
- Revenue up 45% to $65.2 B in FY 2025, with Q4 revenue up 43% and EPS of $7.54 (vs $5.32 in Q4 2024)
- 2026 guidance: Revenue of $80 B–$83 B and EPS of $33.50–$35.00
- Key products contributed over $13 B in Q4, growing 91% year-over-year, led by incretin therapies Mounjaro and Zepbound
- Pipeline progress: submitted Orforglipron in the U.S. and over 40 countries for obesity, and reported positive Phase 3 data for Orforglipron and Retatrutide
- Returned capital: $1.3 B in dividends and $1.5 B in share repurchases
- Revenue of $19.29 B (+42–43% YoY) and non-GAAP EPS of $7.54, both beating expectations
- Mounjaro sales up ~110% (US $4.1 B) and US Zepbound sales up ~122% (US $4.2 B) driving growth
- 2026 guidance raised to $80–83 B in revenue and non-GAAP EPS of $33.50–35.00
- Management highlighted expanded manufacturing capacity and pipeline progress (including orforglipron)
- In Q4 2025, revenue reached $19.3 billion, up 43% year-over-year, driven by volume growth in Mounjaro and Zepbound.
- Q4 2025 EPS was $7.39 reported (+51%) and $7.54 non-GAAP (+42%).
- Full-year 2026 guidance set revenue at $80–$83 billion and non-GAAP EPS at $33.50–$35.00.
- Regulatory progress included FDA approval of Kwikpen for tirzepatide, expanded Jaypirca indication, and submissions for orforglipron in obesity and diabetes.
- In Q4 2025, revenue grew 43% to $19.3 billion and reported EPS rose 51% to $7.39 ($7.54 non-GAAP), driven by volume growth from Mounjaro and Zepbound.
- Mounjaro revenue jumped 110% to $7.4 billion, and Zepbound revenue increased 123% to $4.3 billion in the quarter.
- For full-year 2026, Lilly guided revenue of $80–83 billion and non-GAAP EPS of $33.50–35.00, excluding intangible asset amortization and IPR&D charges.
- Eli Lilly and Zonsen PepLib Biotech Inc. entered a global R&D collaboration and license agreement to develop novel peptide-based drug candidates using PepLib’s proprietary libraries.
- Under the agreement, PepLib will lead screening and identification of optimal peptide molecules, while Lilly will oversee IND-enabling studies, clinical development, and commercialization.
- PepLib will receive upfront and near-term payments, and is eligible for development, regulatory, and sales milestones plus tiered royalties on future net sales.
- The partnership merges PepLib’s peptide discovery platforms with Lilly’s global development and commercialization expertise to advance new therapeutics.
- $3.5 billion investment to build a Lehigh Valley, PA, facility for next-generation weight-loss therapies, including retatrutide.
- Facility will create 850 high-value jobs and generate 2,000 construction jobs, with operations starting in 2031.
- Marks Lilly’s 10th U.S. manufacturing site since 2020 and 4th announced since February 2025, part of over $50 billion in capital commitments to expand U.S. capacity.
Fintool News
In-depth analysis and coverage of ELI LILLY &.

TrumpRx Goes Live: GLP-1 Drug Prices Slashed Up to 85%, Pharma Stocks React

Eli Lilly Crushes Q4 as Mounjaro and Zepbound Sales Double—Stock Surges 8%

Eli Lilly Bets $3.5 Billion on Pennsylvania to Build Retatrutide Arsenal

Eli Lilly Bets $1.12 Billion on Next-Gen Gene Editing for Hearing Loss

Medicare Adds 15 Drugs to Price Negotiation: Biktarvy, Trulicity, Botox on the List

Roche Enters $150 Billion Obesity Drug Race With 22.5% Weight Loss Data
Quarterly earnings call transcripts for ELI LILLY &.
Ask Fintool AI Agent
Get instant answers from SEC filings, earnings calls & more